Copyright
©The Author(s) 2015.
World J Gastroenterol. Feb 14, 2015; 21(6): 1907-1914
Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1907
Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1907
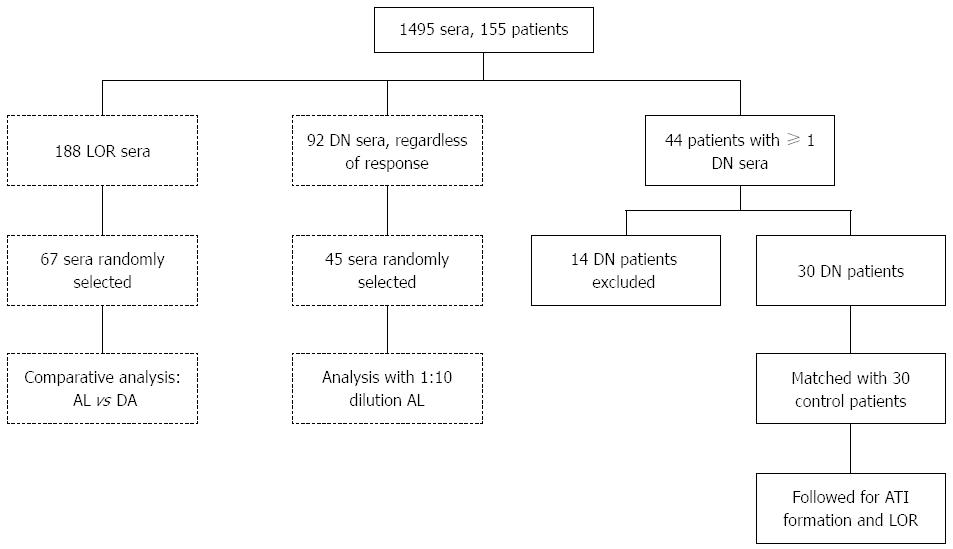
Figure 1 Flow chart of the patients included in the two parts of this study.
The analytical part (dashed lines) comprised a comparison of two different assays and of two different serum dilutions; the clinical part (solid lines) followed up, in a case-control study, double negative patients versus patients with adequate infliximab levels for subsequent antibodies to infliximab formation and clinical outcome. DN: Double negative; LOR: Loss of response; AL: Anti-lambda; DA: Double-antigen; IFX: Infliximab; ATI: Antibodies to infliximab.
- Citation: Ungar B, Anafy A, Yanai H, Ron Y, Yavzori M, Picard O, Fudim E, Loebstein R, Kopylov U, Chowers Y, Dotan I, Eliakim R, Ben-Horin S. Significance of low level infliximab in the absence of anti-infliximab antibodies. World J Gastroenterol 2015; 21(6): 1907-1914
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1907.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1907