Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1900
Peer-review started: July 13, 2014
First decision: August 6, 2014
Revised: September 7, 2014
Accepted: October 14, 2014
Article in press: October 15, 2014
Published online: February 14, 2015
Processing time: 215 Days and 3.3 Hours
AIM: To determine whether fluoroscope time is a good predictor of patient radiation exposure during endoscopic retrograde cholangiopancreatography.
METHODS: This is a prospective observational study of consecutive patients undergoing endoscopic retrograde cholangiopancreatography in a tertiary care setting. Data related to radiation exposure were collected. The following measures were obtained: Fluoroscopy time (FT), dose area product (DAP) and dose at reference point (DOSERP). Coefficients of determination were calculated to analyze the correlation between FT, DAP and DOSRP. Agreement between FT and DAP/DOSRP was assessed using Bland Altman plots.
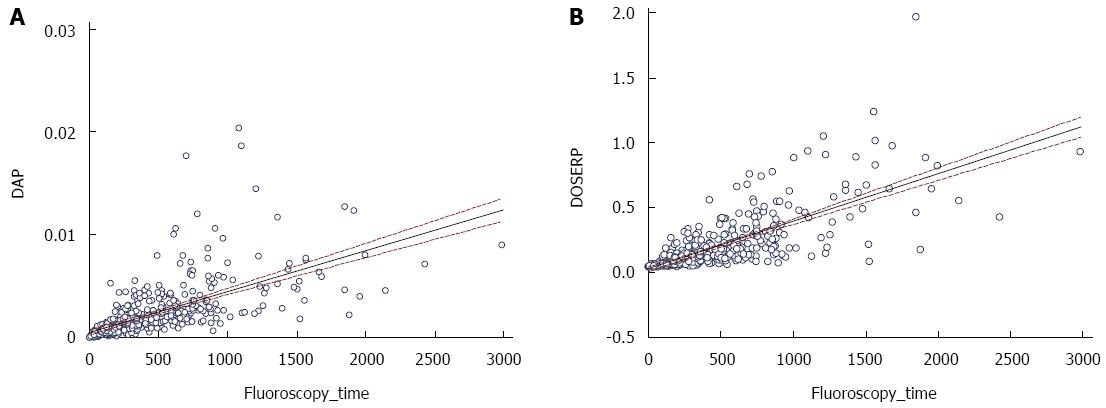
RESULTS: Four hundred sixty-three data sets were obtained. Fluoroscopy time average was 7.3 min. Fluoroscopy related radiation accounted for 86% of the total DAP while acquisition films related radiation accounted for 14% of the DAP. For any given FT there are wide ranges of DAP and DOSERP and the variability in both increases as fluoroscopy time increases. The coefficient of determination (R2) on the non transformed data for DAP and DOSERP versus FT were respectively 0.416 and 0.554. While fluoroscopy use was the largest contributor to patient radiation exposure during endoscopic retrograde cholangiography (ERCP), there is a wide variability in DAP and DOSERP that is not accounted for by FT. DAP and DOSERP increase in variability as FT increases. This translates into poor accuracy of FT in predicting DAP and DOSERP at higher radiation doses.
CONCLUSION: DAP and DOSERP in addition to FT should be adopted as new ERCP quality measures to estimate patient radiation exposure.
Core tip: Endoscopic retrograde cholangiography (ERCP) performance requires endoscopic skills and the use of fluoroscopy with inherent patient and staff radiation exposure. Current ERCP quality measures do not include any measures of radiation. There has been a suggestion to include fluoroscopy time as a radiation quality measure in ERCP. This article provides data on the strength of correlation between fluoroscopy time and more direct measures of radiation exposure such as dose area product and dose at reference point. It also provides a recommendation to include all three measures as quality measures for ERCP. The article presents important principles to achieve the as low as reasonable achievable radiation doses during ERCP.
- Citation: Kachaamy T, Harrison E, Pannala R, Pavlicek W, Crowell MD, Faigel DO. Measures of patient radiation exposure during endoscopic retrograde cholangiography: Beyond fluoroscopy time. World J Gastroenterol 2015; 21(6): 1900-1906
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1900.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1900
Endoscopic retrograde cholangiography (ERCP) is one the most resource intensive complex endoscopic procedures routinely performed. It requires in addition to endoscopic skills the ability to interpret radiologic images in real time. It is also the one with the highest risk. The American Society for Gastrointestinal Endoscopy (ASGE) and the American College of Gastroenterology (ACG) established a task force in 2006 to create quality metrics for endoscopy including ERCP. The quality measures proposed for ERCP are listed in Table 1 and are designed to represent measurable endpoints indicative of high quality care[1].
| Quality indicator |
| 1 Appropriate indication |
| 2 Informed consent |
| 3 Assessment of procedural difficulty |
| 4 Prophylactic antibiotics |
| 5 Cannulation rates |
| Desired duct |
| Use of precut |
| 6 Extraction of common bile duct stones |
| 7 Biliary stent placement |
| 8 Complete documentation |
| 9 Complication rates: pancreatitis, bleeding, perforation, and cholangitis |
The use of fluoroscopy inherent to ERCP results in patients and staff radiation exposure. Since publication of the 2006 guidelines, fluoroscopy time has been proposed as a potential quality metric to add to the original quality measures which did not include a measure of radiation exposure[2]. Radiation exposure in the United States and worldwide have been increasing significantly[3]. Radiation doses to patients and staff during ERCP can be similar to other interventional radiologic procedures[4,5]. While the effects of radiation from one ERCP are unlikely to have any negative effects on patients’ health, the cumulative effects of multiple radiologic procedures including ERCPs can be detrimental. A recent study suggested that radiation doses during ERCP may have declined in recent years partly due to better equipment and partly due to the experience gained in ERCP[6]. We believe that measures of radiation exposure during ERCP need to be included as quality indicators. Good measures should be accurate and easily measured. They should be comparable across centers to compare performance and be included in quality improvement projects. They can help endoscopists become more cognizant of their radiation use and in time reduce patient and staff radiation exposure. The ideal measures would also be comparable to measures in other radiologic procedures as we envision a patient specific radiation exposure measure and ways to minimize radiation exposure as one of the future goals of the healthcare system. Fluoroscopy time has been proposed to be used as a quality measure for ERCP, however, fluoroscopy time is but one of several factors that determine radiation exposure, may not be the most accurate surrogate marker and has its limitations. In fact guidelines for patient radiation dose management from the society of interventional radiology recommend that fluoroscopy time be used with caution to monitor patient radiation doses because of poor correlation with other dose metrics)[7]. This article attempts to define an evidence based quality measure of patient radiation exposure specific to ERCP.
When X-ray energy is absorbed by tissue an electrical charge is produced. In the international system of units (SI) this is measured in Grays. 1 Gray = 1 J/kg. Because different tissues absorb radiation differently the energy produced is tissue dependant. The radiation dose absorbed in humans is measured in tems (radiation equivalent in men) the unit of which in the SI is Sieverts (Sv)[8]. Ionization can cause DNA damage. There are many different forms of radiation related injuries including stochastic and deterministic injuries. In stochastic the probability of an event is related to the amount of exposure but the severity is not; such is the case in cancer induction. Deterministic injuries occur after a certain threshold is reached; an example would be skin related burns[6]. In stochastic injuries there is no amount of radiation which does not lead to possible injury and thus the concept of linear-no-threshold model of radiation exposure. A consequence of this model is the evolution of the concept of using radiation doses as low as reasonably achievable to perform the task or study (ALARA principle). A simplistic estimate of radiation risk from epidemiologic studies suggest that a lifetime exposure to 1 Sv increases the cancer risk by 10% and cancer mortality by 5%[9]. For reference a CT scan exposes the patient to around 10 mSv and translates to an increase by one cancer in every 1000 CT scans. There are many measures of radiation exposure that can be used. Dose Area Product (DAP) is the product of the dose absorbed and the area irradiated and is expressed in Gy square cm. It is an estimation of the entire dose of radiation that the patient receives and is thought to correlate the with long term biologic risk from radiation or stochastic injury. Dose at the reference point (DOSERP) is another measure used and is the dose of radiation delivered to a specific point in space which is, unless otherwise specified, along the central ray 15 cm from the isocenter toward the X-ray tube. DOSERP is relevant to skin injury and deterministic injury. Both can be easily measured by detectors installed on the fluoroscopy unit and the results can be made to be automatically included in reports and transmitted to a database. Because of the ease of measurement, fluoroscopy time has been used as a measure of radiation exposure in ERCP. The assumptions are that FT is a good indicator of radiation exposure and their relationship is linear. However, FT is just one of several factors that determine radiation exposure. These factors include acquisition (spot) films, fluoroscopy pulse rate, patient distance from the x-ray tube, use of oblique imaging, magnification and patient body mass index (BMI). In fact, multiple studies of non-GI interventional radiologic procedures have found FT to be a poor predictor of patient radiation doses such as interventional radiology societies caution against relying exclusively on FT as a measure for patient radiation exposure[5,7,10-12].
A part of an ongoing quality initiative we prospectively collected data from all ERCPs performed in our tertiary care center from January 2012 until June 2013. The following information was obtained: Dose area product (DAP) in milligray meter squared, radiation dose of the reference point in Gray (Gy) DOSERP and fluoroscopy time (FT). DAP and DOSERP data were divided into total, fluoroscopy related and spot films acquisition related: DAPt, DAPf, DAPa, DOSERPt, DOSERPf and DOSERPa respectively. The fluoroscopy unit used was a Siemens unit with the following model and settings: Model - Artis zee multi-purpose stand, Software - VC14J, Detector - Flat panel, Pulse per second - 3 PPS for Fluoroscopy, kVp - 125 kVp for fluoro and 120 kVp for spots (maximum) and mA - 800 mA for fluoro (maximum). 0.1 mm Copper filtration was used in addition to the standard filtration. Radiation meters permanently installed on the fluoroscopy unit provided DAP and DOSRP measurements. Scatter plots were generated. Coefficients of variation were determined on both the transformed and non transformed data. Bland Altman plots were obtained. Statistical analysis was performed using SPSS. The study was exempt from review of the institutional because the data was collected without patient identifiers.
All procedures were performed by three experienced therapeutic endoscopists with the possible involvement of a 4th year advanced endoscopy fellow. Procedures were done in the prone position under general anesthesia. Olympus endoscopic equipment and Boston Scientific short wire rapid exchange accessories were used.
Four hundred sixty-three data sets were obtained. ERCPs were performed by three different attendings.A fellow was involved in approximately 60% of the procedures. The radiation data mean and ranges are shown in Table 2. Fluoroscopy time average was 7.3 min. Fluoroscopy related radiation accounted for 86% of the total DAP while acquisition films related radiation accounted for 14% of the DAP. Every acquisition film was equivalent to approximately 15 seconds of fluoroscopy time (data obtained from a sample of the total data).
| DAPt | DOSERPt | DAPf | DOSERPf | DAPa | DOSERPa | FLUORO_TIME | |
| Mean | 0.0022529 | 0.28213 | 0.1269457 | 0.126946 | 0.000296 | 0.001784 | 7.31 |
| Minimum | 0.0000013 | 0.00004 | 0.0000013 | 0.00004 | 0 | 0 | 1.00 |
| Maximum | 0.004545 | 1.92667 | 0.0042557 | 0.4832 | 0.000289 | 0.0291 | 2141 |
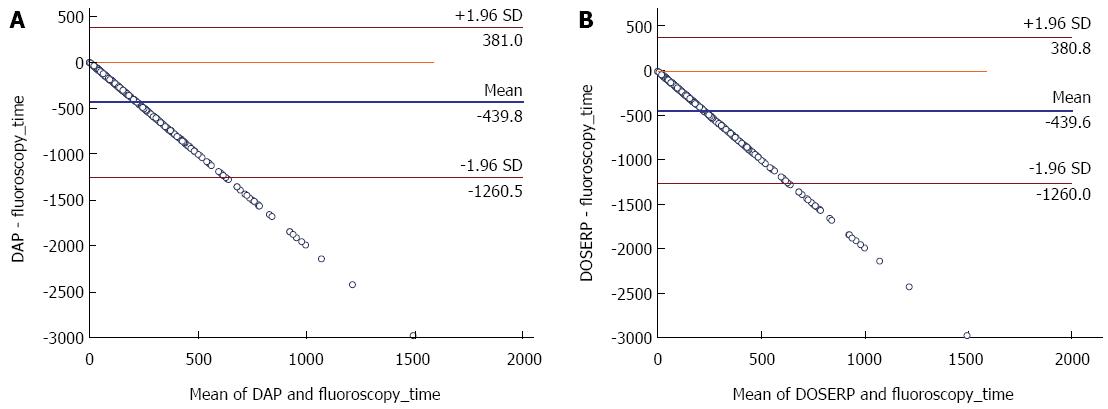
Scatter plots for DAP and DOSERP as a function of FT are shown in Figure 1. The scatter plot show that for any given FT there are wide ranges of DAP and DOSERP and the variability in both increases as fluoroscopy time increases. This is confirmed by the Bland Altman plots where a significant proportional bias was seen and increased as FT increased (Figure 2). The coefficient of variation (R) for DAP versus fluoroscopy time was 0.645. The coefficient of determination (R2) on the non transformed data for DAP and DOSERP versus FT were respectively 0.416 and 0.554. A better linear relationship was found using the log transformed data and the coefficient of variation were 0.66 and 0.69. Data on magnification were available on 183 patients. Changes in magnification accounted for only 6% of the variability in DAP and DOSERP.
The use of fluoroscopy is inherent to ERCP. Since the quality measures proposed by the joint ASGE/ACG task force there has been proposal to include fluoroscopy time as a additional quality indicator to monitor and improve on patient radiation exposure during ERCP[2] . In addition, most studies which looked at patient radiation exposure during ERCP in the past relied on FT. For example a study which looked at factors associated with increased patient exposure used FT as a measure of radiation exposure[13]. Another study which found that radiation exposure during ERCP was lower when providers with more experience performed the procedure used FT as the outcome measured[14]. Other studies looked at the critical determinant of fluoroscopy duration, the effect of training on fluoroscopy duration and the effect of time limited fluoroscopy[15-17]. One very large study which looked at the experience of the endoscopist and “radiation exposure” in ERCP found that more experienced endoscopist used less fluoroscopy time. These studies assumed that radiation exposure strongly correlated with FT[15]. Prior studies which looked at the relationship between DAP and FT had a small number of patients 20, 73 and 54 patients[4,18,19]. A recent study found no correlation between DAP and FT or total number of films taken[20]. A large study which reported fluoroscopy time, DAP and DOSERP found a strong but “not perfect” with an r = 0.728[6]. Our data shows that fluoroscopy use is the largest contributor to patient radiation exposure during ERCP. While there is a good correlation between DAP, DOSERP and FT, there is a wide variability in DAP and DOSERP that is not accounted for by FT. DAP and DOSERP increase in variability as FT increases, and this translates into poor accuracy of FT in predicting DAP and DOSERP where it matters most i.e. at higher radiation doses. Thus, while there is a correlation between FT and exposure, the correlation is not accurate resulting in both under and overestimations of radiation dose if FT were relied upon alone.
DAP and DOSERP reflect multiple other factors not reflected by FT including the patient size and position, the geometry and setting of the fluoroscopy equipment, collimation, angulation, magnification, total number of acquisition films obtained, and radiation filtration. While some factors such as patient size are not controllable by the endoscopist many are modifiable. Some factors can be modified a priori and for all procedures like the equipment settings including pulse per second and filtration. For example changing the pulse rate on the machine from 15 to 3 can decrease patient radiation doses fivefold. This will not be reflected in FT. Copper filtration will filter radiation to the patient which does not contribute to the quality of images and thus decreasing patient radiation exposure without significantly affecting the quality of the image[21]. Some ways to decrease radiation exposure require meticulous attention at the beginning of the procedure like the patient and detector position (Figure 3).
Others ways to decrease radiation doses need behavior modification during the procedure: For instance using last image save instead of acquisition films and using magnification only when it helps significantly in the task being performed and reverting back to the lowest needed magnification once the higher resolution is no longer needed. That being said, in our study magnification had a small effect on total exposure and we believe that the magnification relationship to total radiation exposure is more complex than the obvious. While there is no doubt that magnification increases the amount of radiation per unit time delivered to a point in space, it may involve a smaller radiation field and thus the effect on DAP is complex. In addition, if magnification allows the faster performance of the needed task it might decrease fluoroscopy time with the end result being less radiation than what would be expected purely from magnification. This might explain why magnification was only responsible for 6% of the variation in our data. We would like to emphasize however that magnification does not always lead to better visualization especially if multiple magnification factors are used as the image can become more blurred at higher magnification. This can happen because magnification can alter the focus of the radiation beam on the detector. Suggestions to help the endoscopist follow the ALARA principles are listed in Table 3.
| ALARA principles | |
| Keep the patient away from the radiation source | Use fluorosave instead of acquisition images |
| Keep the detector close to the patient | Keep angulation to a minimum |
| Lower the exposure rate (PPS) | Add 0.1 mm Cu filtration for all protocols |
| Use lowest needed magnification | Step back during acquisition |
| Use collimation | Use personal protective equipment |
| Limit fluoroscopy on-time | Use lead shielding on the fluoroscopy unit |
There are additional benefits for using more direct measures of radiation exposure such as DAP and DOSERP over FT. They are comparable among centers and can be used to establish useful benchmarks for quality improvement. They are also comparable to measures obtained during other imaging procedures and interventional radiologic procedure making a patient centered cumulative radiation measure possible. They will also help the endoscopist collaborate with the radiology department to identify ways to decrease patient and staff radiation exposure beyond just looking at FT. DAP and DOSERP have their limitations. For example they both ignore the radiation delivered to the patient as a result of backscatter. While DOSERP is a good estimate of the skin dose delivered to the patient, the best estimate of skin injury would be the peak skin dose (PSD). PSD represents the highest level of radiation that any part of the skin receives. PSD however is very difficult to measure or determine. Both DAP and DOSERP are easily measured and the values collected can be automated making quality improvement projects easier to implement. In addition current FDA guidelines require any new fluoroscopic unit installed in the United States to have the capability of measuring radiation exposure making this type of quality measure eventually possible for all endoscopists using fluoroscopy. The uncertainties in these measures are currently estimated to be + or -50% for DOSERP and +130% to -70% for FT. DAP uncertainty in measurement is in between these values. While none of these measures are highly accurate in determining the exact amount of radiation exposure, FT is the least accurate[20]. For the above reasons, we believe it is time for endoscopists performing ERCP on regular basis to join other fluoroscopy based disciplines in monitoring their patient radiation exposure by incorporating DAP and DOSERP measurements in addition to FT as part of their quality measures.
Patient radiation exposure is increasing in the United States and worldwide. Patient radiation exposure during ERCP can be similar to other interventional radiologic procedures. Quality measures reflecting patient radiation exposure during ERCP are needed. Based on the above data, we recommend adopting DAP and DOSERP in addition to FT as new ERCP quality measures to estimate patient radiation exposure.
Endoscopic retrograde cholangiography (ERCP) is one of the most complicated gastrointestinal procedures routinely performed. It requires in addition to endoscopic skills the use of fluoroscopy with inherent patient and staff radiation exposure. Quality measures were proposed in 2006 by a joint the American Society of Gastrointestinal endoscopy and the American College of Gastroenterology task force but did not include any measures of radiation. Since the publication of these quality measures fluoroscopy time has been proposed to be added as a measure reflecting patient radiation exposure.
Studies of fluoroscopy time correlation with patient radiation exposure during ERCP are small. Interventional radiology and cardiology literature suggest that fluoroscopy time is an inaccurate measure of radiation exposure. Well designed large studies looking at ERCP radiation quality measures are lacking.
This is the largest study on radiation measures in ERCP. The findings are contrary to prior small studies which showed that fluoroscopy time is an excellent measure of patient radiation exposure. The findings are consistent with other fluoroscopy based disciplines and recommendation of interventional radiology societies.
The authors recommend using Dose Area Product and Dose at Reference Point in addition to Fluroroscopy time as measures of patient radiation exposure. These measures will allow creation of ERCP specific radiation benchmarks which can be comparable among centers. They will also allow the possibility of tracking total radiation dose for a given patient across disciplines including diagnostic imaging.
Dose area product (DAP): is a surrogate marker of the radiation risk to the tissue irradiated. It is the product of the radiation dose absorbed and the area irradiated expressed in gray cm square. It does not account for the radiation dose cause by scatter. It is easily measured by placing a dosimeter beyond the collimator in a way to intercept the radiation beam. DAP correlates with the risk of stochastic effects such as cancer induction. Dose at reference point (DOSRP): is the dose of radiation delivered to a specific point in space which is, unless otherwise specified, along the central ray 15 cm from the isocenter toward the x-ray tube. It does not include radiation related to backscatter. DOSRP correlates with the risk of deterministic effects such as skin injury. Peak Skin dose: is the highest radiation dose received by any part of the patient skin. This includes radiation from the primary X-ray bean and backscatter. It is difficult to measure but is the best estimate of deterministic effects. Stochastic effects: a radiation effect whose probability of occurrence is related to the amount of exposure but the severity is not; such is the case in cancer induction. Deterministic effects: a radiation effect whose probability occurs after a certain threshold is reached; an example would be skin related burns.
This is a very interesting prospective and descriptive study. The authors have been able to show that fluoroscopy time is not an accurate indirect measurement of the radiation exposure during the ERCP procedure to the patient and medical staff.
P- Reviewer: Rabago L, Singhal S S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Baron TH, Petersen BT, Mergener K, Chak A, Cohen J, Deal SE, Hoffman B, Jacobson BC, Petrini JL, Safdi MA. Quality indicators for endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 2006;63:S29-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Romagnuolo J, Cotton PB. Recording ERCP fluoroscopy metrics using a multinational quality network: establishing benchmarks and examining time-related improvements. Am J Gastroenterol. 2013;108:1224-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Mettler FA, Bhargavan M, Faulkner K, Gilley DB, Gray JE, Ibbott GS, Lipoti JA, Mahesh M, McCrohan JL, Stabin MG. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources--1950-2007. Radiology. 2009;253:520-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 584] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 4. | Buls N, Pages J, Mana F, Osteaux M. Patient and staff exposure during endoscopic retrograde cholangiopancreatography. Br J Radiol. 2002;75:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Brambilla M, Marano G, Dominietto M, Cotroneo AR, Carriero A. Patient radiation doses and references levels in interventional radiology. Radiol Med. 2004;107:408-418. [PubMed] |
| 6. | Rodríguez-Perálvarez ML, Miñano-Herrrero JA, Hervás-Molina AJ, Benítez-Cantero JM, García-Sánchez V, Naranjo-Rodríguez A, Pleguezuelo-Navarro M, Soler-Cantos Mdel M, de la Mata-García M. Radio induced cancer risk during ERCP. Is it a real clinical problem? Rev Esp Enferm Dig. 2011;103:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Stecker MS, Balter S, Towbin RB, Miller DL, Vañó E, Bartal G, Angle JF, Chao CP, Cohen AM, Dixon RG. Guidelines for patient radiation dose management. J Vasc Interv Radiol. 2009;20:S263-S273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 325] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 8. | Campbell N, Sparrow K, Fortier M, Ponich T. Practical radiation safety and protection for the endoscopist during ERCP. Gastrointest Endosc. 2002;55:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Royal HD. Effects of low level radiation-what’s new? Semin Nucl Med. 2008;38:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Panuccio G, Greenberg RK, Wunderle K, Mastracci TM, Eagleton MG, Davros W. Comparison of indirect radiation dose estimates with directly measured radiation dose for patients and operators during complex endovascular procedures. J Vasc Surg. 2011;53:885-894.e1; discussion 894. [PubMed] |
| 11. | Weiss DJ, Pipinos II, Longo GM, Lynch TG, Rutar FJ, Johanning JM. Direct and indirect measurement of patient radiation exposure during endovascular aortic aneurysm repair. Ann Vasc Surg. 2008;22:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Mohapatra A, Greenberg RK, Mastracci TM, Eagleton MJ, Thornsberry B. Radiation exposure to operating room personnel and patients during endovascular procedures. J Vasc Surg. 2013;58:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Boix J, Lorenzo-Zúñiga V. Radiation dose to patients during endoscopic retrograde cholangiopancreatography. World J Gastrointest Endosc. 2011;3:140-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Gonzalez-Gonzalez JA, Martínez-Vazquez MA, Maldonado-Garza HJ, Garza-Galindo AA. Radiation doses to ERCP patients are significantly lower with experienced endoscopists. Gastrointest Endosc. 2011;73:415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Kim E, McLoughlin M, Lam EC, Amar J, Byrne M, Telford J, Enns R. Prospective analysis of fluoroscopy duration during ERCP: critical determinants. Gastrointest Endosc. 2010;72:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Uradomo LT, Lustberg ME, Darwin PE. Effect of physician training on fluoroscopy time during ERCP. Dig Dis Sci. 2006;51:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Uradomo LT, Goldberg EM, Darwin PE. Time-limited fluoroscopy to reduce radiation exposure during ERCP: a prospective randomized trial. Gastrointest Endosc. 2007;66:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Larkin CJ, Workman A, Wright RE, Tham TC. Radiation doses to patients during ERCP. Gastrointest Endosc. 2001;53:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Heyd RL, Kopecky KK, Sherman S, Lehman GA, Stockberger SM. Radiation exposure to patients and personnel during interventional ERCP at a teaching institution. Gastrointest Endosc. 1996;44:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Tsapaki V, Paraskeva KD, Mathou N, Andrikopoulos E, Tentas P, Triantopoulou C, Karagiannis JA. Patient and endoscopist radiation doses during ERCP procedures. Radiat Prot Dosimetry. 2011;147:111-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Fetterly KA. Investigation of the practical aspects of an additional 0.1 mm copper x-ray spectral filter for cine acquisition mode imaging in a clinical care setting. Health Phys. 2010;99:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |