Published online Dec 28, 2015. doi: 10.3748/wjg.v21.i48.13593
Peer-review started: April 27, 2015
First decision: September 29, 2015
Revised: November 6, 2015
Accepted: November 24, 2015
Article in press: November 24, 2015
Published online: December 28, 2015
Major duodenal papilla cancer (MDPC) represents the primary type of duodenal cancer, and is typically considered a periampullary carcinoma as most tumors arise in this region. This report describes an extremely rare case involving a patient with rapidly and extensively recurrent MDPC following pancreaticoduodenectomy, who achieved complete response by concurrent image-guided radiation and intravenous oxaliplatin plus oral capecitabine therapies. The patient was a 50-year-old female who was admitted to our hospital 6 wk after resection for MDPC for evaluation of a nontender and enlarged node in the left side of her neck. After clinical work-up, the patient was diagnosed with postoperatively recurrent MDPC with widespread lymph node metastases at the bilateral cervix, mediastinum, abdominal cavity, and retroperitoneal area. She was administered whole field image-guided radiation therapy along with four cycles of the intravenous oxaliplatin plus oral capecitabine regimen. A complete response by positron emission tomography with 18-fluorodeoxyglucose was observed 4 months after treatment. The patient continues to be disease-free 2 years after the diagnosis of recurrence.
Core tip: Major duodenal papilla cancer (MDPC) is a rare malignancy, and there are limited data regarding its recurrence after radical resection. This report describes a case of recurrent MDPC with widespread lymph node involvement at the bilateral cervix, mediastinum, abdominal cavity, and retroperitoneal area, 6 wk after pancreaticoduodenectomy. The patient experienced a complete response to image-guided radiation therapy and a concomitant regimen of intravenous oxaliplatin plus oral capecitabine, and remains disease-free 2 years after the diagnosis of recurrence. This, to our knowledge, is the first case to demonstrate the role of chemoradiotherapy with improved survival in extensively recurrent MDPC.
- Citation: Li BS, Shi H, Wen M, Xiao MY, Wang J. Widespread lymph node recurrence of major duodenal papilla cancer following pancreaticoduodenectomy. World J Gastroenterol 2015; 21(48): 13593-13598
- URL: https://www.wjgnet.com/1007-9327/full/v21/i48/13593.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i48.13593
Major duodenal papilla cancer (MDPC), despite representing approximately 60% of all small bowel malignancies, remains a very rare disease that comprises less than 0.5% of all gastrointestinal cancers[1]. The primary treatment is surgical resection, with pancreaticoduodenectomy or Whipple procedure as the preferred approach for resectable lesions[2]. However, recurrent disease following radical surgery has been inadequately documented. Particularly, there is very little data in the literature regarding the treatment of relapse settings involving multiple lymph node (LN) metastases. This report describes a case involving widespread LN recurrence of an adenocarcinoma of the major duodenal papilla after pancreaticoduodenectomy in which complete response was achieved by image-guided radiation therapy (IGRT) and a concomitant regimen of intravenous oxaliplatin plus oral capecitabine (XELOX).
An otherwise healthy, 50-year-old Chinese female had previously consulted another hospital in January 2013 with significant upper abdominal pain. At that time, she underwent a pancreaticoduodenectomy and lymphadenectomy, and was diagnosed intraoperatively with MDPC. Postoperative pathology revealed a well-differentiated adenocarcinoma of the major duodenal papilla. Surgical margins and four LNs were negative for tumor tissue. The patient’s postoperative course was uneventful and she was discharged 2 wk after the operation.
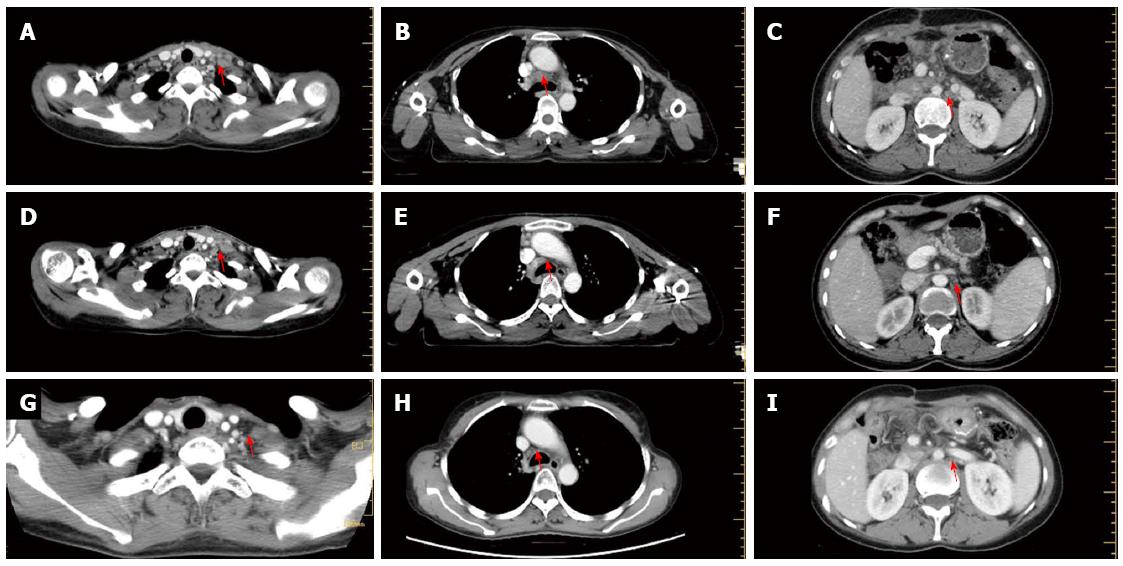
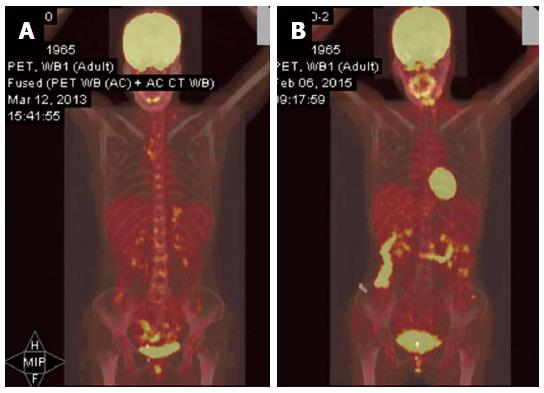
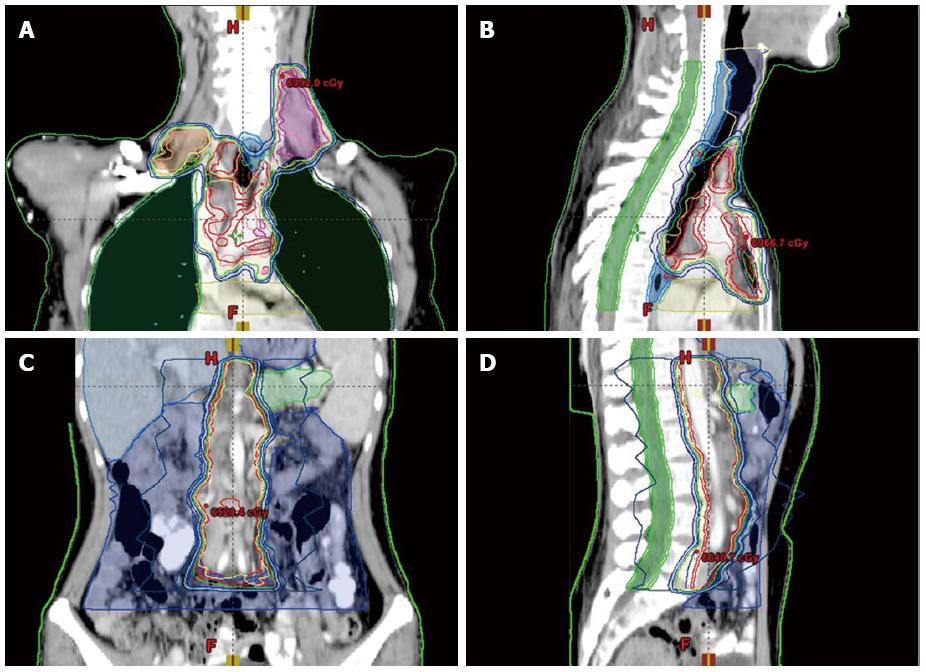
Six weeks following the surgery, in March 2013, she became aware of a hard, palpable nodule in the left supraclavicular area, and was referred to our hospital. Upon initial examination, she had no clinical symptoms, such as hoarseness, abdominal pain, or melena. The Karnofsky performance status was 90%. Physical examination of the cervical region showed a fixed, nontender, left supraclavicular LN, approximately 2 cm in diameter. A total body computed tomography (CT) scan revealed swollen LNs at the bilateral cervix, mediastinum, abdominal cavity, and retroperitoneal area (Figure 1A-C). At this time, the patient’s serum level of carbohydrate antigen (CA)19-9 was 1046 U/mL (normal range, < 34 U/mL). Metastatic adenocarcinoma was confirmed by ultrasonographic-guided fine-needle biopsy of the left neck. Subsequent fluorodeoxyglucose-positron emission tomography/CT (PET/CT) fusion imaging revealed hypermetabolic foci at the above-mentioned sites with mean standard uptake values of 2.8 (bilateral cervix), 2.4 (mediastinum), 1.8 (abdominal cavity) and 1.8 (retroperitoneal area) (Figure 2A). Based on these findings, postoperative widespread LN recurrence of MDPC was the likely diagnosis. After multidisciplinary consultation, a multimodality therapeutic strategy was planned consisting of whole-field IGRT and four concomitant cycles of the XELOX regimen [intravenous oxaliplatin (130 mg/m2) on day 1 and oral capecitabine (750 mg/m2) twice daily on days 1-14 every 4 wk]. Specifically, the dose prescriptions of IGRT were 6444 cGy/25 plus a boost of 1745 cGy/7 fractions to the left cervical nodes, 6170 cGy/25 plus a boost of 1754 cGy/7 fractions to the right cervical nodes, 6170 cGy/25 plus a boost of 1730 cGy/7 fractions to the mediastinal nodes, and 5744 cGy/25 fractions to the celiac nodes. The dosimetric values for target coverage and organ-at-risk sparing were defined according to the criteria of the International Commission on Radiation Units and Measurements guidelines. The gross tumor volume was defined as the resection site and the suspicious LNs visualized on CT imaging, including the bilateral cervical, mediastinal, and retroperitoneal areas (Figure 3). Prescribed doses were the minimum for the gross tumor volume. The outlined organs at risk were the uninvolved bilateral lungs, esophagus, spinal cord, bilateral kidneys, and small bowel (Figure 3). IGRT planning was performed using the Varian Medical Systems Eclipse 11.0.47 (Varian Medical Systems, Inc., Palo Alto, CA, United States). There were no interruptions or delays in chemoradiotherapy.
One and a half months after treatment, a re-evaluation with CT scan was carried out. Remarkably, the patient achieved a near complete remission (Figure 1D-F), and her serum CA19-9 level returned to normal.
The patient completed chemoradiotherapy in late July 2013. No other adverse effects with clinical significance were observed, except grade 3 diarrhea and grade 2 skin reactions. A subsequent 2-year follow-up showed no evidence of disease recurrence (Figure 1G-I). Furthermore, a recent fluorodeoxyglucose-PET examination showed almost total disappearance of the known uptake areas (Figure 2B).
Recurrent disease after curative resection represents a major challenge for effective treatment of MDPC, which has a median survival of less than 1 year after the diagnosis of recurrence[3,4]. These studies also report a median time to tumor recurrence of 14.5-29 mo, with locoregional (tumor bed and regional LNs) and/or distant (liver, peritoneum, lung, and supraclavicular LNs) recurrence patterns. The predominant site of relapse is the operative bed, followed by the liver and retroperitoneal LNs. Widespread metastatic LN failure, however, is extremely rare[5,6]. Moreover, there are limited reports concerning treatment decisions for cases of recurrent MDPC following pancreaticoduodenectomy, especially for rapid, widespread LN recurrence. The current case involves a patient who experienced early and extensive LN recurrence along with an elevated CA19-9 level less than 2 mo following surgery. Complete response was achieved with concurrent therapies of IGRT and a XELOX regimen. This, to our knowledge, is the first case to demonstrate the role of chemoradiotherapy and a corresponding survival improvement in extensively recurrent MDPC.
Given the rare occurrence of MDPC, the role of chemotherapy, radiotherapy, or combined chemoradiotherapy remains unclear, particularly for recurrent disease after surgery. For patients with inoperable or metastatic small bowel adenocarcinoma (SBA), chemotherapy can improve overall survival when compared with no chemotherapy[4,7-9]. These studies used regimens of capecitabine combined with oxaliplatin (XELOX or CAPOX), or administered them as an adjuvant therapy after curative or palliative surgery for patients with MDPC[10]. Pharmacologically, capecitabine is a prodrug of the cytotoxic agent 5-FU, which selectively exerts its anticancer effects preferentially in tumor cells via the up-regulation of thymidine phosphorylase. Furthermore, capecitabine is orally administered and clinically tolerable during the treatment process. The combination of capecitabine with oxaliplatin reduces the need for intravenous drug administration and associated visits to the clinic. As advanced and recurrent MDPC are similar in tumor biology, the XELOX regimen was selected for treatment of the patient in the case presented here.
The role of radiation therapy (RT) is also not well defined. Available studies are either retrospective or involve a small number of patients, and report either only a slight increase in 1-year survival or no significant survival advantage for patients receiving adjuvant RT after resection of primary tumors[2,11]. Nevertheless, retrospective studies have also reported high rates of in-field control when adjuvant RT was administered to high-risk patients with positive margins, LN metastases, and locally aggressive tumor biology[5]. Thus, there is general agreement on the value of RT in reducing local failure of MDPC following radical resection. IGRT has emerged as a safe and effective technique to precisely deliver conformal RT in real time, while sparing critical organs[12,13]. Indeed, successful use of this technique in other malignancies indicated an additional benefit of IGRT in the treatment of recurrent MDPC[14,15]. Consequently, IGRT was chosen concurrent with the XELOX regimen to treat the patient described in this case report, which had a successful outcome as observed by her survival for more than 2 years from the diagnosis of recurrence.
Some side effects have previously been reported for the same therapies used to treat the patient in the present case. These include diarrhea, nausea, vomiting, fatigue, abdominal pain, hand-foot syndrome, and peripheral sensory neuropathy for the XELOX regimen, and subcutaneous fibrosis, edema, and joint stiffness for IGRT[16]. Indeed, the patient in the present case experienced grade 3 diarrhea due to the oral capecitabine, which required temporary parenteral nutrition. Additionally, acute grade 2 dermatitis and pneumonitis occurred due to IGRT, though the patient tolerated these adverse effects well without discontinuing treatment.
The current AJCC guideline recommends that six is the minimum number of LNs to be examined for duodenal cancer, including MDPC, though it has been questioned whether the threshold should be raised[17]. Recent data from the Surveillance, Epidemiology, and End Results database demonstrated that increasing the number of dissected LNs correlates to improved survival in stage II SBA[18]. Other studies also reported that the removal of at least 15 LNs improved prognostic discrimination by the pathologic N category, and that increasing the total number of LNs assessed markedly improved prognostication for patients with stage I, II, and III SBA who underwent resection[18,19]. The patient in the present case had only four LNs dissected, which may explain the rapid and widespread recurrence of the disease. Nevertheless, there are also molecular mechanisms that may be associated with the aggressiveness of invasion and metastasis early in the course of the disease[20].
Another aspect worth mentioning is the sharply increased level of the tumor marker CA19-9, which, in general, is significantly associated with a diagnosis of pancreatic and colorectal adenocarcinoma[21,22]. However, there is less data concerning the correlation between serum CA19-9 levels and postoperative survival in patients with SBA, though a retrospective multicenter study found that serum CA19-9 values were independent prognostic factors for progression-free and overall survival of such patients[23]. It is unclear whether the CA19-9 concentration is a prognostic indicator in patients with MDPC, though it can potentially be used to survey for the recurrence of MDPC. As observed in our patient, there was a sudden rise in CA19-9 levels 40 d postoperatively, which may have been indicative of the widespread recurrence of disease.
In conclusion, our experience demonstrates that XELOX with concomitant IGRT is a potential treatment for MDPC patients with widespread LN recurrence after radical pancreaticoduodenectomy. Therefore, future studies should further evaluate the efficacy of the regimen in this very rare setting.
We appreciate Professor Qiang Li for the critical reading of the manuscript and fruitful discussion.
A 50-year-old female with recurrent major duodenal papilla cancer (MDPC) following pancreaticoduodenectomy.
Postoperatively recurrent MDPC with widespread lymph node metastases at the bilateral cervix, mediastinum, abdominal cavity, and retroperitoneal area.
Periampullary cancers (pancreas, duodenum, distal common bile duct, and ampulla of Vater).
Serum carbohydrate antigen 19-9 was 1046 U/mL (normal range, < 34 U/mL).
Positron emission tomography with 18-fluorodeoxyglucose/computed tomography showed hypermetabolic foci at the bilateral cervix, mediastinum, abdominal cavity, and retroperitoneal sites with mean standard uptake values of 2.8, 2.4, 1.8 and 1.8 respectively.
Metastatic adenocarcinoma was confirmed by ultrasonographic-guided fine-needle biopsy of the left neck.
Intravenous oxaliplatin plus oral capecitabine regimen concurrent with image-guided radiation therapy.
MDPC is typically described as periampullary carcinoma because most tumors arise in the periampullary region.
A regimen of intravenous oxaliplatin plus oral capecitabine concomitant with image-guided radiation therapy is a potential treatment for MDPC patients with widespread lymph node recurrence after radical pancreaticoduodenectomy.
In a case presentation, the authors wrote that a patient was operated on for major duodenal papilla cancer. The paper is interesting and innovating.
P- Reviewer: Shiryajev YN S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Kim MJ, Choi SB, Han HJ, Park PJ, Kim WB, Song TJ, Suh SO, Choi SY. Clinicopathological analysis and survival outcome of duodenal adenocarcinoma. Kaohsiung J Med Sci. 2014;30:254-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Schmidt CM, Powell ES, Yiannoutsos CT, Howard TJ, Wiebke EA, Wiesenauer CA, Baumgardner JA, Cummings OW, Jacobson LE, Broadie TA. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg. 2004;139:718-725; discussion 725-727. [PubMed] [Cited in This Article: ] |
| 3. | Cecchini S, Correa-Gallego C, Desphande V, Ligorio M, Dursun A, Wargo J, Fernàndez-del Castillo C, Warshaw AL, Ferrone CR. Superior prognostic importance of perineural invasion vs. lymph node involvement after curative resection of duodenal adenocarcinoma. J Gastrointest Surg. 2012;16:113-20; discussion 120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518-526. [PubMed] [Cited in This Article: ] |
| 5. | Kim K, Chie EK, Jang JY, Kim SW, Oh DY, Im SA, Kim TY, Bang YJ, Ha SW. Role of adjuvant chemoradiotherapy for duodenal cancer: a single center experience. Am J Clin Oncol. 2012;35:533-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Poultsides GA, Huang LC, Cameron JL, Tuli R, Lan L, Hruban RH, Pawlik TM, Herman JM, Edil BH, Ahuja N. Duodenal adenocarcinoma: clinicopathologic analysis and implications for treatment. Ann Surg Oncol. 2012;19:1928-1935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Overman MJ, Varadhachary GR, Kopetz S, Adinin R, Lin E, Morris JS, Eng C, Abbruzzese JL, Wolff RA. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27:2598-2603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Fishman PN, Pond GR, Moore MJ, Oza A, Burkes RL, Siu LL, Feld R, Gallinger S, Greig P, Knox JJ. Natural history and chemotherapy effectiveness for advanced adenocarcinoma of the small bowel: a retrospective review of 113 cases. Am J Clin Oncol. 2006;29:225-231. [PubMed] [Cited in This Article: ] |
| 9. | Swartz MJ, Hughes MA, Frassica DA, Herman J, Yeo CJ, Riall TS, Lillemoe KD, Cameron JL, Donehower RC, Laheru DA. Adjuvant concurrent chemoradiation for node-positive adenocarcinoma of the duodenum. Arch Surg. 2007;142:285-288. [PubMed] [Cited in This Article: ] |
| 10. | Ynson ML, Senatore F, Dasanu CA. What are the latest pharmacotherapy options for small bowel adenocarcinoma? Expert Opin Pharmacother. 2014;15:745-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Kelsey CR, Nelson JW, Willett CG, Chino JP, Clough RW, Bendell JC, Tyler DS, Hurwitz HI, Morse MA, Clary BM. Duodenal adenocarcinoma: patterns of failure after resection and the role of chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;69:1436-1441. [PubMed] [Cited in This Article: ] |
| 12. | Wang D, Zhang Q, Eisenberg BL, Kane JM, Li XA, Lucas D, Petersen IA, DeLaney TF, Freeman CR, Finkelstein SE. Significant Reduction of Late Toxicities in Patients With Extremity Sarcoma Treated With Image-Guided Radiation Therapy to a Reduced Target Volume: Results of Radiation Therapy Oncology Group RTOG-0630 Trial. J Clin Oncol. 2015;33:2231-2238. [PubMed] [Cited in This Article: ] |
| 13. | De Los Santos J, Popple R, Agazaryan N, Bayouth JE, Bissonnette JP, Bucci MK, Dieterich S, Dong L, Forster KM, Indelicato D. Image guided radiation therapy (IGRT) technologies for radiation therapy localization and delivery. Int J Radiat Oncol Biol Phys. 2013;87:33-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Szeto A, Chin L, Whelan P, Wilson J, Lee J. Image-guided radiation therapy using surgical clips for localization of colonic metastasis from thyroid cancer. Radiat Oncol. 2014;9:298. [PubMed] [Cited in This Article: ] |
| 15. | Monroe AT, Pikaart D, Peddada AV. Clinical outcomes of image guided radiation therapy (IGRT) with gold fiducial vaginal cuff markers for high-risk endometrial cancer. Acta Oncol. 2013;52:1010-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | O’Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S, Pitot HC, Shields AF, Landry JC, Ryan DP. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014;32:1927-1934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 300] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 17. | Cloyd JM, Norton JA, Visser BC, Poultsides GA. Does the extent of resection impact survival for duodenal adenocarcinoma? Analysis of 1,611 cases. Ann Surg Oncol. 2015;22:573-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Overman MJ, Hu CY, Wolff RA, Chang GJ. Prognostic value of lymph node evaluation in small bowel adenocarcinoma: analysis of the surveillance, epidemiology, and end results database. Cancer. 2010;116:5374-5382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Sarela AI, Brennan MF, Karpeh MS, Klimstra D, Conlon KC. Adenocarcinoma of the duodenum: importance of accurate lymph node staging and similarity in outcome to gastric cancer. Ann Surg Oncol. 2004;11:380-386. [PubMed] [Cited in This Article: ] |
| 20. | Alwmark A, Andersson A, Lasson A. Primary carcinoma of the duodenum. Ann Surg. 1980;191:13-18. [PubMed] [Cited in This Article: ] |
| 21. | Boone BA, Steve J, Zenati MS, Hogg ME, Singhi AD, Bartlett DL, Zureikat AH, Bahary N, Zeh HJ. Serum CA 19-9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann Surg Oncol. 2014;21:4351-4358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 22. | Stiksma J, Grootendorst DC, van der Linden PW. CA 19-9 as a marker in addition to CEA to monitor colorectal cancer. Clin Colorectal Cancer. 2014;13:239-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Zaanan A, Costes L, Gauthier M, Malka D, Locher C, Mitry E, Tougeron D, Lecomte T, Gornet JM, Sobhani I. Chemotherapy of advanced small-bowel adenocarcinoma: a multicenter AGEO study. Ann Oncol. 2010;21:1786-1793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |