Copyright
©The Author(s) 2015.
World J Gastroenterol. Nov 7, 2015; 21(41): 11777-11792
Published online Nov 7, 2015. doi: 10.3748/wjg.v21.i41.11777
Published online Nov 7, 2015. doi: 10.3748/wjg.v21.i41.11777
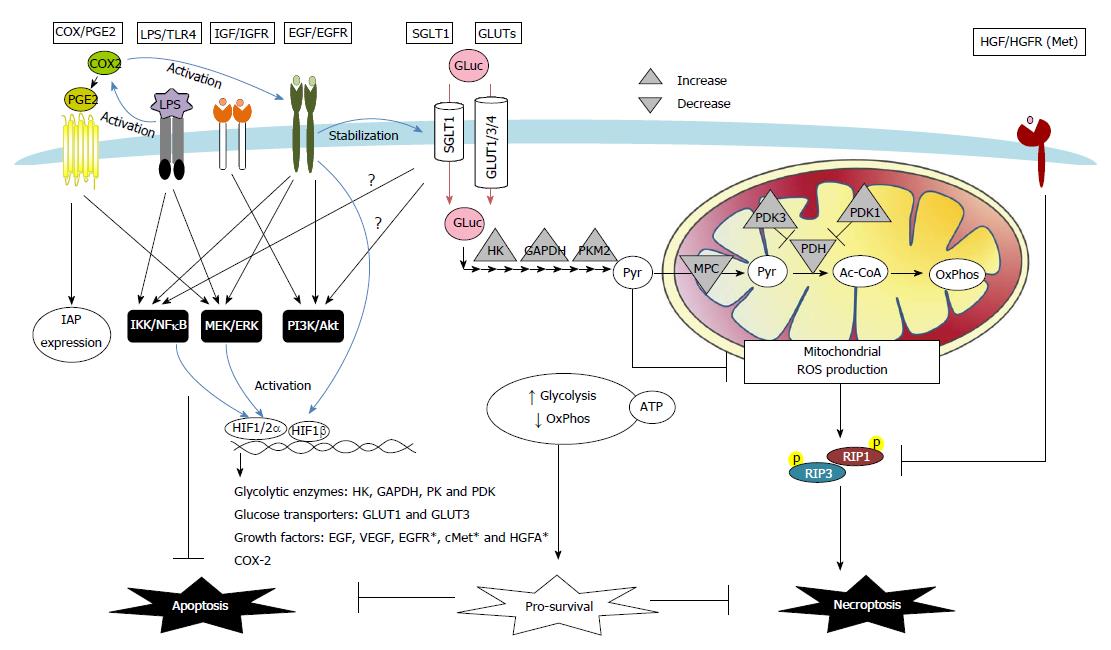
Figure 2 Proposed schema of death desistance mechanisms via modulation of receptor signaling and transporter uptake in colon cancer cells.
A number of autocrine, paracrine, or exogenous factors instigate death resistance in colon carcinoma. These pathways included cyclooxygenase (COX)-2/prostaglandin E2 (PGE2), bacterial lipopolysaccharide (LPS)/Toll-like receptor 4 (TLR4), growth factors [i.e., insulin-like growth factor (IGF), epidermal growth factor (EGF), and hepatocyte growth factor (HGF)], as well as glucose transport and metabolism. COX/PGE2 upregulates the IAP expression and activates EGF/EGFR signaling to inhibit apoptosis in colon cancer cells. TLR4 antagonizes cell apoptosis caused by its co-receptor CD14, induces anti-apoptotic MEK/ERK and IKK/IκB/NFκB signaling, and activates COX-2 pathways in colon carcinoma. Growth factors such as IGF and EGF induce anti-apoptotic PI3K/Akt, MEK/ERK, and IKK/IκB/NFκB pathways in colon cancers. Moreover, activation of HGF and its receptor Met renders colon cancer cells resistant to necroptosis via downregulation of RIPK1 protein expression. Alteration of transport and metabolism of glucose (Gluc) is another survival strategy of cancer cells. Abnormally expressed sodium-dependent glucose transporter 1 (SGLT1) and GLUT1/3/4 enhance glucose uptake in colon carcinoma. Activation of SGLT1 induces PI3K/Akt and IKK/IκB/NFκB pathways in normal intestinal epithelial cells; however, their roles in anti-death mechanisms of colon cancers remain unclear (?). Increased glycolysis and decreased mitochondria-dependent oxidative phosphorylation (OxPhos) are commonly seen in cancer cells. The metabolic shift results from upregulated expression of glycolytic enzymes for increased (Pyr) production [e.g., hexokinase (HK), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), pyruvate kinase (PK)], and also from downregulated expression of mitochondrial pyruvate carrier (MPC) and pyruvate dehydrogenase (PDH) that limits pyruvate conversion to Acetyl Co-A (Ac-CoA). The metabolic shift and predominantly glycolytic ATP generation are adaptive responses to hypoxic stress and promotes cancer cell survival. The final glycolytic product pyruvate, which is also a free radical scavenger, prevents hypoxia-induced necroptotic death in colon cancer cells via suppression of mitochondrial ROS. Hypoxia acts a stressor but also a death regulator by HIF-dependent transcription of a number of genes, including glucose metabolic enzymes [e.g., HK, GAPDH, PK, and pyruvate dehydrogenase kinase (PDK)], glucose transporters (e.g., GLUT-1 and GLUT-3), and growth factors [e.g., EGF and vascular endothelial growth factors (VEGF)]. Other HIF-targeted genes, e.g., EGFR, cMet, and HGF activator (HGFA), were reported on non-intestinal cancer cells (*), and may also contribute to the death resistance mechanisms. Lastly, EGF activates HIF signaling in normoxic conditions, leading to a positive feedback loop of adaptation fueling anti-death and pro-proliferative cancer growth.
- Citation: Huang CY, Yu LCH. Pathophysiological mechanisms of death resistance in colorectal carcinoma. World J Gastroenterol 2015; 21(41): 11777-11792
- URL: https://www.wjgnet.com/1007-9327/full/v21/i41/11777.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i41.11777