Copyright
©The Author(s) 2015.
World J Gastroenterol. Oct 28, 2015; 21(40): 11396-11410
Published online Oct 28, 2015. doi: 10.3748/wjg.v21.i40.11396
Published online Oct 28, 2015. doi: 10.3748/wjg.v21.i40.11396
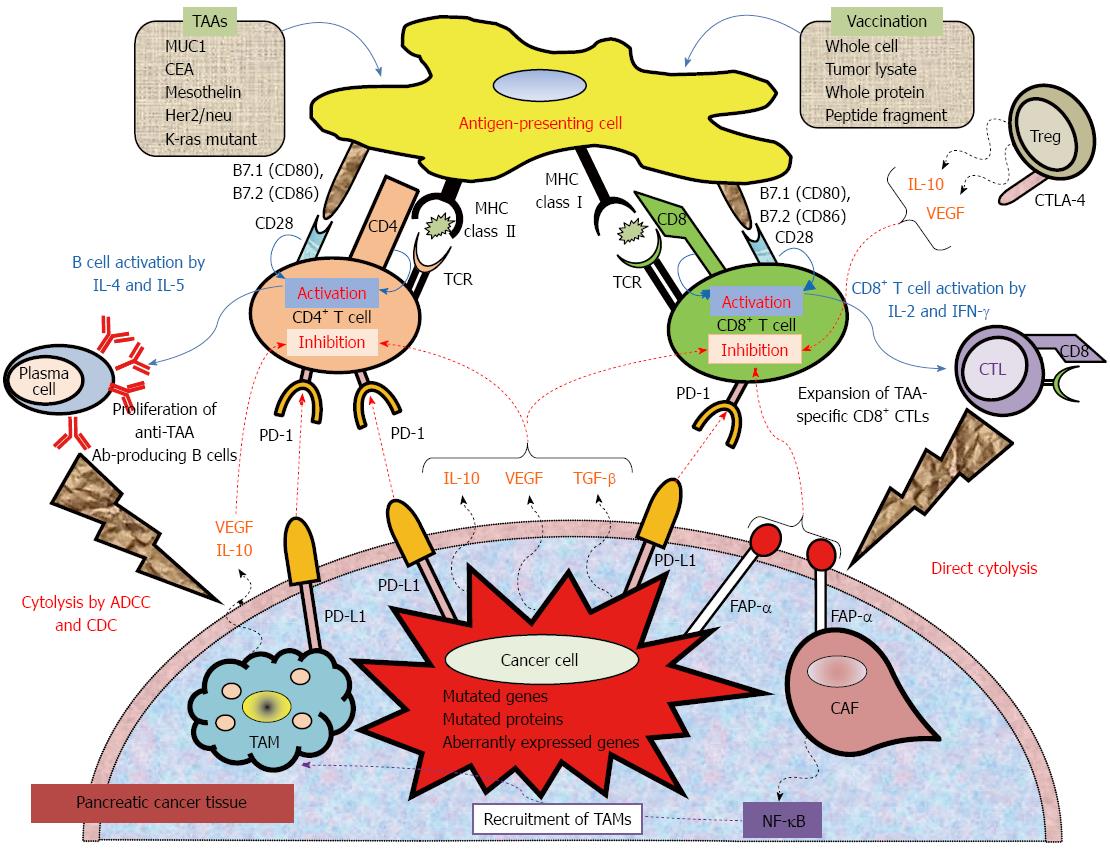
Figure 1 Mechanistic basis for vaccine-based immunotherapy, and immune co-stimulatory, co-inhibitory ligands and receptors, and soluble immune modulating factors involved in T cell activation and inhibition.
Anticancer immunotherapy aims to harness the natural ability of the immune system to recognize and react against potential TAAs. Peptide-based, protein-based, or whole cell-based vaccines rely on identified immune-dominant TAA epitopes to stimulate anticancer T-cell responses. Antigen-presenting cells (APCs), including dendritic cells, capture antigens obtained from vaccinations. After intracellular processing, antigen peptides are loaded onto major histocompatibility complex (MHC class I or II) molecules on the surface of the APC. Specific T cells encounter these MHC-peptide complexes in conjugation with a co-stimulatory signal. The activated T cells proliferate and secrete cytokines (IL-2, IL-4, IL-5, INF-γ), resulting in the production of a cascade of immune effector cells. Immunotherapeutic strategies that inhibit immune checkpoints such as those mediated by CTLA-4 and PD-1 reduce the barriers that vaccines must overcome to trigger therapeutically relevant anticancer immune responses. Recently, preclinical and clinical studies have demonstrated that combining immune-modulating agents such as cyclophosphamide (CY) and checkpoint inhibitors (anti-CTLA-4, anti-PD-1, anti-PD-L1) with vaccine strategies can enhance anticancer immune responses as well as block the tolerizing mechanisms that would otherwise inhibit these responses. ADCC: Antibody-dependent cell-mediated cytotoxicity; CAF: Cancer-associated fibroblast; CDC: Complement-dependent cytolysis; CTLA-4: Cytotoxic T lymphocyte antigen-4; FAP-α: Fibroblast activation protein-α; TAM: Tumor-associated macrophage; TCR: T cell receptor; PD-1: Programmed cell death protein 1; PD-L1: Programmed death-ligand.
- Citation: Tanemura M, Miyoshi E, Nagano H, Eguchi H, Matsunami K, Taniyama K, Hatanaka N, Akamatsu H, Mori M, Doki Y. Cancer immunotherapy for pancreatic cancer utilizing α-gal epitope/natural anti-Gal antibody reaction. World J Gastroenterol 2015; 21(40): 11396-11410
- URL: https://www.wjgnet.com/1007-9327/full/v21/i40/11396.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i40.11396