Published online Jan 28, 2015. doi: 10.3748/wjg.v21.i4.1222
Peer-review started: May 26, 2014
First decision: July 9, 2014
Revised: August 16, 2014
Accepted: October 15, 2014
Article in press: October 15, 2014
Published online: January 28, 2015
Processing time: 248 Days and 22.4 Hours
AIM: To evaluate survival data in patients with gastric cancer in relation to postoperative adjuvant therapy and survival determinants
METHODS: A total of 201 patients (mean ± SD age: 56.0 ± 11.9 years, 69.7% were males) with gastric carcinoma who were operated and followed up at Lutfi Kirdar Kartal Training and Research Hospital between 1998 and 2010 were included in this retrospective study. Follow up was evaluated divided into two consecutive periods (before 2008 and 2008-2010, respectively) based on introduction of 3-D conformal technique in radiotherapy at our clinic in 2008. Data on patient demographics, clinical and histopathological characteristics of gastric carcinoma and the type of treatment applied after surgery [postoperative adjuvant treatment protocols including chemoradiotherapy (CRT) and chemotherapy (CT), supportive therapy or follow up without any treatment] were recorded. The median duration and determinants of local recurrence free (LRF) survival, distant metastasis free (DMF) survival and overall survival were evaluated in the overall population as well as with respect to follow up years [1998-2008 (n = 127) vs 2008-2010 (n = 74)].
RESULTS: Median duration for LRF survival, DMF survival and overall survival were 31.9, 24.1 and 31.9 mo, respectively in patients with postoperative adjuvant CRT. No significant difference was noted in median duration for LRF survival, DMF survival and overall survival with respect to treatment protocols in the overall population and also with respect to followed up periods. In the overall population, CT protocols FUFA [5-fluorouracil (400 mg/m2) and leucovorin-folinic acid (FA, 20 mg/m2)] (29.9 mo) and UFT® + Antrex® [a fixed combination of the oral FU prodrug tegafur (flouroprymidine, FT, 300 mg/m2 per day) with FA (Antrex®), 15 mg tablet, two times a day] (42.5 mo) was significantly associated with longer LRF survival times than other CT protocols (P = 0.036), while no difference was noted between CT protocols in terms of DMF survival and overall survival. Among patients received CRT, overall survival was significantly longer in patients with negative than positive surgical margin (27.7 mo vs 22.4 mo, P = 0.016) in the overall study population, while time of radiotherapy initiation had no significant impact on survival times. Nodal stage was determined to be independent predictor of LRF survival in the overall study population with 4.959 fold (P = 0.042) increase in mortality in patients with nodal stage N2 compared to patients with nodal stage N0, and independent predictor of overall survival with 5.132 fold (P = 0.006), 5.263 fold (P = 0.027) and 4.056 fold (P = 0.009) increase in the mortality in patients with nodal stage N3a (before 2008), N3b (before 2008) and N2 (overall study population) when compared to patients with N0 stage, respectively.
CONCLUSION: Our findings emphasize the likelihood of postoperative adjuvant CRT to have a survival benefit in patients with resectable gastric carcinoma.
Core tip: This retrospective single centre analysis of survival data in patients with resected gastric carcinoma revealed median 31.9 mo of local recurrence free (LRF) survival, 24.1 mo of distant metastasis free survival and 31.9 mo of overall survival via postoperative adjuvant chemoradiotherapy during follow up from 1998 to 2010. Use of 5-fluorouracil and leucovorin-folinic acid and uracil/tegafur based chemotherapy protocols and the absence of positive surgical margin but not the interval between surgery and radiotherapy had a significant impact on survival times, while the nodal stage was the independent prognostic factor for LRF and overall survival.
- Citation: Ozden S, Ozgen Z, Ozyurt H, Gemici C, Yaprak G, Tepetam H, Mayadagli A. Survival in gastric cancer in relation to postoperative adjuvant therapy and determinants. World J Gastroenterol 2015; 21(4): 1222-1233
- URL: https://www.wjgnet.com/1007-9327/full/v21/i4/1222.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i4.1222
Despite advances in surgical techniques, patients with gastric cancer show poor prognosis and cure rate remains dismal with 5-year survival rates of 8%-34% and locoregional recurrence of 40%-90% even after curative resection[1].
Accordingly implication of neoadjuvant or adjuvant therapy in patients with resectable gastric cancer mainly in the form of postoperative chemoradiotherapy (CRT) and perioperative chemotherapy (CT) has been considered by several studies in terms achievement of better therapeutic outcomes and shown to be associated with high-level evidence for improved survival in Western populations[2-5].
Besides, based on data from the phase III, INT 0116-SWOG0008 study in which better survival rates were achieved by adding CT (5-fluorouracil and leucovorin-folinic acid) and concurrent 45 Gy radiotherapy to surgery[2], postoperative CRT has become a standard in gastric carcinoma, especially in United States[6].
Additionally, a past meta-analysis and the Surveillance, Epidemiology, and End Results database have demonstrated a favorable survival impact of radiotherapy in patients with resectable gastric cancer[7,8]. However, despite increasing evidence available for a survival advantage from adjuvant therapies, adjuvant treatment strategies in patients with resectable gastric cancer still remains debated[9,10] particularly in terms of favour of radiotherapy associated with CT, the adequacy of nodal dissection, the likelihood of CT related toxic effects and inconsistency of different therapeutic trials in terms of survival and relapse rates[11-13].
Given that radiotherapy has been included as a component of adjuvant therapy at our institution, the present single-centre retrospective study (1998-2010) was designed to analyze survival data in patients with gastric cancer after surgical resection in relation to efficacy of postoperative adjuvant therapy protocols and survival determinants.
A total of 201 patients (mean ± SD age: 56.0 ± 11.9) years, 69.7% were male with gastric cancer who were operated and followed up at Lutfi Kirdar Kartal Training and Research Hospital between 1998 and 2010 were included in this retrospective study. In order to prevent the likelihood of misinterpretation of survival outcome, follow up was evaluated divided into two consecutive periods (before 2008 and 2008-2010, respectively) based on introduction of 3-D conformal technique in radiotherapy in 2008. All patients who were operated due to gastric cancer with stage T3 or T4 and/or any T level with positive lymph node (stage IB-IIIC) were included in the study except for 2 patients who had radiotherapy per se as the post adjuvant treatment.
While the present study was exempt from the requirement of ethical approval in relation to its retrospective design, the permission was obtained from our institutional ethics committee for the use of patient data for publication purposes.
Data on patient demographics, clinical and histopathological characteristics of gastric carcinoma and the type of treatment applied after surgery (postoperative adjuvant treatment protocols including CRT, CT, supportive therapy or follow up without any treatment) were recorded and the rate and determinants of local recurrence free (LRF) survival, distant metastasis free (DMF) survival and overall survival were evaluated in the overall population as well as with respect to follow up years [1998-2008 (n = 127) vs 2008-2010 (n = 74)].
Clinical staging was performed according to American Joint Committee on Cancer (AJCC) Staging Manual, Sixth Edition (2002 and 2010), published by Springer Science + Business Media. Details of tumour site, histology and stage were recorded, as was the type of surgical resection on the basis of histopathological reports. Thorax and total abdominal computed tomography, complete blood counts including liver and renal function tests and bone scan if elevated alkaline phosphotase or bone pain present were performed for distant metastasis evaluation.
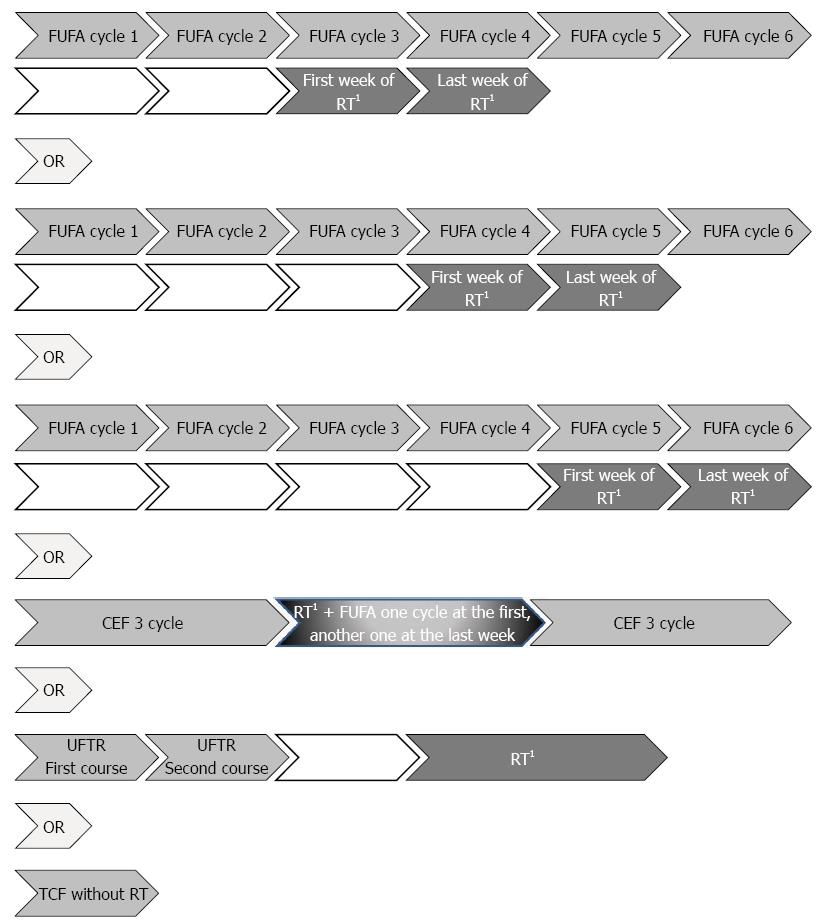
Figure 1 illustrates the schema of postoperative chemoradiotherapy. The CT regimen involved 1-2 cycles of bolus FUFA [5-fluorouracil (5-FU, 400 mg/m2 per day) and leucovorin-folinic acid (FA, 20 mg/m2) D1-5 every 28 d]. Usually third and fourth or fourth and fifth cycles of FUFA or fifth and sixth cycles of FUFA according to performance status of patient and patient waiting list for machine, were applied concomitantly during first and last weeks of RT course, remaining cycles of CT were given in 4 wk after the completion of radiotherapy or CEF [(cisplatin 50 mg/m2), eprubicin (50 mg/m2) and 5-FU (500 mg/m2), D1 every 21 d], followed by 45 Gy simulator planned concurrent radiotherapy in 25 daily fractions of for 5 wk. For CEF regime, usually after 3 cycles, concomitantly FUFA was applied during first and last week of radiotherapy the same as FUFA regime, after completion of CRT, remaining 3 cycles of CEF was applied. At 3 weekly intervals 14 d of UFTR [a fixed combination of the oral FU prodrug tegafur (flouroprymidine, FT, 300 mg/m2 per day in two divided doses) with FA (Antrex®), 15 mg tablet, two times a day] was given for two to three course and then the same doses with radiotherapy throughout the whole radiotherapy course excluding weekends for 5 wk. Few patients were applied TCF regime without radiotherapy including TCF (docetaxel 75 mg/m2 in combination with cisplatin 75 mg/m² on Day 1 and fluorouracil 750 mg/m2 per day by continuous infusion for five days).
Two dimensional (2D) treatment was applied with Saturn 41, 1996, France, GE using plan Target 2; 3D treatments were applied with Siemens Onco impression 2007, with XiO planning system, 2007 or DHX, varian, United States, eclips planning, 2007. Fields included tumor site, residual stomach and peripheral lymph nodes. All the rules of RTOG for organ at risks were strictly obeyed, no overdose was used.
After completion of treatments every three months for two years, 6 mo up to 5 years, annually afterwards, controls of patients included physical examination, whole blood counts liver and renal function tests, tumor markers carcinoembryonic antigen and carbohydrate antigen 19-9, total abdominal ultrasonography or magnetic resonance imaging, chest X-ray when patient has any complaints.
Statistical analysis was made using MedCalc Statistical Software version 12.7.7 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2013), χ2 and Fisher-Exact tests were used for the comparison of categorical data, while numerica data were analyzed using Student-t test and Mann-Whitney U test for variables with normal distribution and for non-normally distributed variables, respectively. Survival analysis was made via Kaplan Meier analysis and comparisons were made via Log-Rank test. Correlates of survival were determined via Cox-Regression analysis with inclusion of independent variables with P < 0.2 significance in the univariate analysis into the model via Hosmer-Lemeshow method. Data were expressed as “mean ± SD”, minimum-maximum and percent (%) where appropriate. P < 0.05 was considered statistically significant.
Adenocarcinoma (65.2%) was the most common histological type; the tumor was poorly differentiated in 64.2% of patients and located in the antrum in 47.2% with T3T4 stage in 68.7% and AJCC 2002 nodal stage of N1 in 48.3% and AJCC 2010 nodal stage of N1 in 21.9% (Table 1).
| Follow up (yr) | Total (n = 201) | P value | ||
| Before 2008 (n = 127) | 2008-2010 (n = 74) | |||
| Demographics | ||||
| Age (yr), mean ± SD | 56.7 ± 12.7 | 54.7 ± 10.3 | 56.0 ± 11.9 | 0.2701 |
| Gender | ||||
| Female | 36 (28.3) | 25 (33.8) | 61 (30.3) | 0.4192 |
| Male | 91 (71.7) | 49 (66.8) | 140 (69.7) | |
| Clinicopathologic characteristics | ||||
| Pathological type | ||||
| AdenoCa | 92 (72.4) | 39 (52.7) | 131 (65.2) | 0.0012 |
| Signet ring | 33 (26.0) | 31 (41.9) | 64 (31.8) | |
| Squamous | 2 (1.6) | 0 | 0 | |
| Mucinous | 0 | 4 (5.4) | 4 (2.0) | |
| Tumor size | ||||
| < 5 cm | 49 (43.0) | 28 (47.5) | 77 (44.5) | 0.0552 |
| 5-10 cm | 60 (52.6) | 23 (39.0) | 83 (48.0) | |
| > 10 cm | 5 (4.4) | 8 (13.6) | 13 (7.5) | |
| Tumor location | ||||
| Antrum | 56 (44.8) | 37 (51.4) | 93 (47.2) | 0.2492 |
| Cardia | 29 (23.2) | 15 (20.8) | 44 (22.3) | |
| Corpus | 37 (29.6) | 15 (20.8) | 52 (26.4) | |
| > 1 region | 3 (2.4) | 5 (6.9) | 8 (4.1) | |
| Differentiation | ||||
| Well | 2 (1.7) | 1 (1.8) | 3 (1.7) | 0.8852 |
| Moderate | 41 (35.3) | 18 (31.6) | 59 (34.1) | |
| Poor | 73 (62.9) | 38 (66.7) | 111 (64.2) | |
| Surgery type | ||||
| Total gastrectomy | 64 (50.4) | 37 (50.0) | 101 (50.2) | 0.9572 |
| Subtotal gastrectomy | 63 (49.6) | 37 (50.0) | 100 (49.8) | |
| Surgical margin | ||||
| Negative | 114 (89.8) | 61 (82.4) | 175 (87.1) | 0.1352 |
| Positive | 13 (10.2) | 13 (17.6) | 26 (12.9) | |
| Vascular involvement | 81 (64.8) | 58 (80.6) | 139 (70.6) | 0.0333 |
| Perineural involvement | 80 (64.0) | 52 (72.2) | 132 (68.4) | 0.4263 |
| TM stage | ||||
| T1T2 | 40 (31.5) | 23 (31.1) | 63 (31.3) | 0.9512 |
| T3T4 | 87 (68.5) | 51 (68.9) | 138 (68.7) | |
| Nodal stage | ||||
| N0 | 30 (24.2) | 12 (16.7) | 42 (21.4) | 0.2542 |
| N1 | 55 (44.4) | 26 (36.1) | 81 (41.3) | |
| N2 | 26 (21.0) | 24 (33.3) | 50 (25.5) | |
| N3 | 13 (10.5) | 10 (13.9) | 23 (11.7) | |
| AJCC 2002 nodal stage | ||||
| N0 | 32 (25.2) | 14 (18.9) | 46 (22.9) | 0.3642 |
| N1 | 62 (48.8) | 35 (47.3) | 97 (48.3) | |
| N2 | 26 (20.5) | 15 (20.3) | 41 (20.4) | |
| N3 | 6 (4.7) | 8 (10.8) | 14 (7.0) | |
| NX | 1 (0.8) | 2 (2.7) | 3 (1.5) | |
| AJCC 2010 nodal stage | ||||
| N0 | 32 (25.2) | 14 (18.9) | 46 (22.9) | 0.2312 |
| N1 | 32 (25.2) | 12 (16.2) | 44 (21.9) | |
| N2 | 31 (24.4) | 23 (31.1) | 54 (26.9) | |
| N3a | 25 (19.7) | 15 (20.3) | 40 (19.9) | |
| N3b | 6 (4.7) | 8 (10.8) | 14 (7.0) | |
| Nx | 1 (0.8) | 2 (2.7) | 3 (1.5) | |
| Dissected lymph nodes, median (IQR) | 15 (13.0) | 17.5 (13.0) | 16 (14.0) | 0.2164 |
| LN1 | ||||
| < 15 | 66 (52.4) | 30 (40.5) | 96 (48.0) | 0.1062 |
| ≥ 16 | 60 (47.6) | 44 (59.5) | 104 (52.0) | |
| LN2 | ||||
| < 10 | 38 (30.2) | 18 (24.3) | 56 (28.0) | 0.3752 |
| ≥ 11 | 88 (69.8) | 56 (75.7) | 144 (72.0) | |
| Involved lymph nodes, median (IQR) | 2 (6.0) | 4 (7.0) | 3 (6.0) | 0.0734 |
In patients followed up before 2008, adenocarcinoma type (72.4% vs 52.7%, P = 0.001) was more common and vascular involvement (64.8% vs 80.6%, P = 0.033) was less common than in patients followed up after 2008, while demographic and other clinicopathological characteristics were similar between the two groups (Table 1).
CRT was the leading postoperative treatment applied in 73.1% of overall patients and more commonly in the follow up period of 2008-2010 compared to follow up before 2008 (85.1% vs 66.1%, P = 0.023). CT per se, supportive treatment and follow up without treatment were more prevalent in the follow up before 2008 compared with later years (Table 2).
| Treatment | Follow up (yr) | Total (n = 201) | P value1 | |
| Before 2008 (n = 127) | 2008-2010 (n = 74) | |||
| Chemoradiotherapy | 84 (66.1) | 63 (85.1) | 147 (73.1) | 0.023 |
| Chemotherapy | 19 (15.0) | 6 (8.1) | 25 (12.4) | |
| Supportive treatment | 10 (7.9) | 2 (2.7) | 12 (6.0) | |
| None (follow up) | 14 (11.0) | 3 (4.1) | 17 (8.5) | |
| Radiotherapy | 0.003 | |||
| No | 43 (33.9) | 11 (14.9) | 54 (26.9) | |
| Yes | 84 (66.1) | 63 (85.1) | 147 (73.1) | |
| Chemotherapy protocol | < 0.001 | |||
| FUFA | 70 (55.1) | 42 (56.8) | 112 (55.7) | |
| CEF | 9 (7.1) | 21 (28.3) | 30 (14.9) | |
| None | 14 (11.0) | 3 (4.1) | 17 (8.5) | |
| UFT | 23 (18.1) | 1 (1.4) | 24 (11.9) | |
| TCF | 1 (0.8) | 5 (6.8) | 6 (3.0) | |
| Supportive | 10 (7.9) | 2 (2.7) | 12 (6.0) | |
| Type of radiotherapy device | < 0.001 | |||
| Co60 | 21 (25.3) | 0 (0.0) | 21 (14.4) | |
| Linak | 58 (69.9) | 7 (11.1) | 65 (44.5) | |
| Varian-Siemens | 4 (4.8) | 56 (88.9) | 60 (41.1) | |
| Simulator planning | < 0.001 | |||
| 2 dimensional | 79 (62.7) | 7 (9.5) | 86 (43.0) | |
| 3 dimensional | 4 (3.2) | 56 (75.7) | 60 (30.0) | |
| No radiotherapy | 43 (34.1) | 11 (14.9) | 54 (27.0) | |
FUFA (55.7% in the overall population, 55.1% before 2008, and 56.8% in 2008-2010) was the most commonly applied CT protocol regardless of the follow up period, as followed by CEF (14.9%) and UFT (11.9%). CEF in patients followed up in 2008-2010 (28.3% vs 7.1%, P < 0.001) and UFT in patients followed up before 2008 (18.1% vs 1.4%, P < 0.001) were more common CTs (Table 2).
Considering radiotherapy use of Co60 or Lineer accelerator 6-15 MV photon with 2-D simulator planning (62.7%) before 2008, while use of 6-23 MV photon lineer accelaretors with 3-D simulator planning (75.7%) after 2008 were more common (P < 0.001, Table 2).
Median duration for LRF survival, DMF survival and overall survival were 31.9, 24.1 and 31.9 mo, respectively in patients who received postoperative adjuvant CRT. Local recurrence occurred in 27 (13.7%) patients during the entire follow up and in 18 (14.4%) and 9 (12.5%) patients in the follow up periods of 1998-2008 and 2008-2010, respectively.
No significant difference was noted in median duration for LRF survival, DMF survival and overall survival with respect to treatment protocols during the entire follow up period as well as in patients followed up before or after 2008 (Table 3).
| Total (n = 201) | Before 2008 (n = 127) | 2008-2010 (n = 74) | |||||||
| LFS | DFS | OS | LFS | DFS | OS | LFS | DFS | OS | |
| Treatment protocols | |||||||||
| Chemoradiotherapy | 31.9 | 24.1 | 31.9 | 37.8 | 24.1 | 37.8 | 11.7 | 19.2 | 11.7 |
| Chemotherapy | 25.9 | 20.6 | 27.1 | 43.6 | 22.3 | 51.7 | 15.0 | 18.2 | 15.3 |
| P value1 | 0.793 | 0.834 | 0.597 | 0.792 | 0.656 | 0.630 | 0.959 | 0.848 | 0.715 |
| Chemotherapy protocolsa | |||||||||
| FUFA | 29.9 | 23.8 | 31.9 | ||||||
| UFT | 42.5 | 20.6 | 53.0 | ||||||
| CEF | 13.3 | 16.0 | 13.3 | ||||||
| TCF | 15.0 | 1.6 | 15.0 | ||||||
| P value (FUFA-UFT)1 | 0.036 | 0.6 | 0.477 | ||||||
| Surgical margin2 | |||||||||
| Positive | 20.6 | 21.4 | 22.4 | 43.8 | 20.5 | 52.2 | 15.0 | 19.1 | 15.0 |
| Negative | 26.0 | 19.2 | 27.7 | 41.5 | 48.2 | 50.9 | 15.0 | 18.3 | 15.3 |
| P value1 | 0.509 | 0.511 | 0.016 | 0.239 | 0.126 | 0.053 | 0.519 | 0.699 | 0.185 |
| RT simulator planningb | |||||||||
| 2 dimensional | 42.4 | 23.1 | 50.4 | ||||||
| 3 dimensional | 14.1 | 16.0 | 14.1 | ||||||
| P value3 | NA | NA | NA | ||||||
| Time of RT initiation | |||||||||
| < 4 mo | 36.3 | 20.6 | 40.9 | ||||||
| ≥ 4 mo | 39.8 | 16.8 | 39.8 | ||||||
| P value | 0.0581 | NA | 0.3704 | ||||||
In the overall population, CT protocols FUFA (29.9 mo) and UFT (42.5 mo) were significantly associated with longer median duration for LRF survival than CEF (13.3 mo) and TCF (15.0 mo) (P = 0.036 for each), while no difference was noted between CT protocols in terms of DMF survival and overall survival (Table 3). Among patients received CRT, overall survival was significantly longer in patients with negative than positive surgical margin (27.7 mo vs 22.4 mo, P = 0.016), while the interval between surgery and radiotherapy had no significant impact on survival times (Table 3).
In the univariate analysis, vascular involvement (P = 0.005 in follow up before 2008), AJCC 2002 nodal stage (P = 0.024 in overall study population) and the number of involved lymph nodes (P = 0.002 in follow up before 2008 and P < 0.001 in the overall study population) were significantly associated with LRF survival (Table 4).
| Local recurrence free survival (mo) | Distant metastasis free survival (mo) | Overall survival (mo) | |||||||
| Before 2008 (n = 127) | 2008-2010 (n = 74) | Total (n = 201) | Before 2008 (n = 127) | 2008-2010 (n = 74) | Total (n= 201) | Before 2008 (n = 127) | 2008-2010 (n = 74) | Total (n = 201) | |
| Age | |||||||||
| ≤ 50 yr | 44.1 | 20.6 | 49.8 | 14.0 | 23.1 | 14.0 | 28.7 | 20.6 | 33.7 |
| > 50 yr | 39.1 | 26.0 | 43.6 | 15.9 | 18.3 | 15.9 | 26.0 | 22.2 | 26.7 |
| P value | 0.066 | 0.471 | 0.312 | 0.747 | 0.925 | 0.48 | 0.044 | 0.65 | 0.181 |
| Type of treatment | |||||||||
| Chemoradiotherapy | 37.8 | 24.1 | 37.8 | 11.7 | 19.2 | 11.7 | 31.9 | 24.1 | 31.9 |
| Chemotherapy | 43.6 | 22.3 | 51.7 | 15.0 | 18.2 | 15.3 | 25.9 | 20.6 | 27.1 |
| P value | 0.792 | 0.656 | 0.63 | 0.959 | 0.848 | 0.715 | 0.793 | 0.834 | 0.597 |
| Type of gastrectomy surgery | |||||||||
| Total | 39.8 | 15.9 | 26.7 | 29.8 | 18.3 | 24.0 | 43.9 | 15.9 | 29.1 |
| Subtotal | 43.3 | 13.8 | 25.9 | 14.9 | 19.1 | 16.0 | 46.9 | 13.8 | 28.5 |
| P value | 0.885 | 0.122 | 0.888 | 0.395 | 0.636 | 0.393 | 0.318 | 0.1 | 0.092 |
| Vascular involvement | |||||||||
| No | 49.7 | 11.5 | 39.7 | 26.0 | - | 26.0 | 49.8 | 11.5 | 43.9 |
| Yes | 35.0 | 16.0 | 23.5 | 24.0 | 18.3 | 21.4 | 37.8 | 16.2 | 24.0 |
| P value | 0.697 | 0.005 | 0.594 | 0.858 | - | 0.81 | < 0.001 | 0.795 | < 0.001 |
| Perineural involvement | |||||||||
| No | 45.9 | 14.9 | 31.3 | 24.0 | - | 24.0 | 48.6 | 14.9 | 32.5 |
| Yes | 41.2 | 15.0 | 25.0 | 22.4 | 16.0 | 20.5 | 43.6 | 15.2 | 25.9 |
| P value | 0.706 | 0.353 | 0.822 | 0.609 | - | 0.999 | 0.122 | 0.275 | 0.058 |
| T stage | |||||||||
| T1 | 55.4 | 20.2 | 48.6 | 27.6 | - | 27.6 | 55.4 | 20.2 | 48.6 |
| T2 | 54.4 | 16.4 | 28.3 | 29.4 | 30.2 | 29.8 | 54.4 | 16.9 | 32.4 |
| T3 | 37.0 | 14.1 | 25.1 | 24.1 | 18.3 | 22.1 | 41.1 | 14.1 | 26.0 |
| T4 | 30.9 | 24.4 | 24.4 | 18.6 | 12.6 | 16.6 | 31.8 | 30.6 | 30.6 |
| P value | 0.054 | 0.959 | 0.128 | 0.668 | 0.223 | 0.353 | 0.04 | 0.28 | 0.002 |
| Nodal stage | |||||||||
| N0 | 54.9 | 13.7 | 45.7 | 41.1 | 22.2 | 41.1 | 54.9 | 14.8 | 47.8 |
| N1 | 46.9 | 14.6 | 29.1 | 30.6 | 21.0 | 27.7 | 53.7 | 14.8 | 37.0 |
| N2 | 23.2 | 16.0 | 19.2 | 16.6 | 18.3 | 16.6 | 24.1 | 16.0 | 19.2 |
| N3 | 27.1 | 13.4 | 16.6 | 16.1 | 12.6 | 15.0 | 35.0 | 13.4 | 20.7 |
| P value | 0.515 | 0.504 | 0.647 | 0.02 | 0.887 | 0.014 | < 0.001 | 0.439 | < 0.001 |
| Nodal stage (AJCC 2002) | |||||||||
| N0 | 54.9 | 11.3 | 42.1 | 41.1 | 22.2 | 41.1 | 54.9 | 11.3 | 45.7 |
| N1 | 42.4 | 16.9 | 28.3 | 30.6 | 20.5 | 26.0 | 49.2 | 16.9 | 29.1 |
| N2 | 19.3 | 15.5 | 16.1 | 14.2 | 13.8 | 14.2 | 20.1 | 15.5 | 19.1 |
| N3 | 31.0 | 10.7 | 15.0 | 19.5 | 19.8 | 19.8 | 31.0 | 10.7 | 15.0 |
| P value | 0.057 | 0.283 | 0.024 | 0.011 | 0.817 | 0.001 | < 0.001 | 0.224 | < 0.001 |
| LN1 | |||||||||
| < 15 | 40.5 | 14.9 | 30.5 | 24.0 | 19.1 | 23.1 | 45.7 | 14.9 | 33.2 |
| > 16 | 42.7 | 15.0 | 25.4 | 24.0 | 18.3 | 20.5 | 44.9 | 15.2 | 25.7 |
| P value | 0.181 | 0.768 | 0.423 | 0.309 | 0.742 | 0.664 | 0.724 | 0.461 | 0.523 |
| LN2 | |||||||||
| < 10 | 34.0 | 15.9 | 25.4 | 20.9 | 22.2 | 22.2 | 40.5 | 16.2 | 31.0 |
| > 11 | 46.4 | 14.6 | 26.8 | 24.1 | 16.0 | 22.2 | 47.8 | 14.6 | 28.0 |
| P value | 0.169 | 0.768 | 0.373 | 0.204 | 0.742 | 0.349 | 0.985 | 0.464 | 0.822 |
| Nodal stage (AJCC 2010) | |||||||||
| N0 | 54.9 | 11.3 | 42.1 | 41.1 | 22.2 | 41.1 | 54.9 | 11.3 | 45.7 |
| N1 | 51.0 | 16.9 | 39.1 | 29.4 | 36.8 | 33.1 | 53.8 | 16.9 | 44.9 |
| N2 | 32.5 | 16.0 | 25.8 | 28.0 | 18.3 | 22.3 | 37.8 | 17.0 | 25.9 |
| N3a | 19.6 | 15.0 | 16.1 | 15.4 | 13.8 | 14.6 | 20.7 | 15.0 | 17.7 |
| N3b | 31.0 | 15.0 | 16.3 | 19.5 | 19.8 | 19.8 | 31.0 | 15.0 | 16.3 |
| P value | 0.131 | 0.402 | 0.057 | 0.06 | 0.849 | 0.039 | < 0.001 | 0.098 | < 0.001 |
| Involved lymph nodes (n) | |||||||||
| ≤ 5 | 48.6 | 35.5 | 52.2 | 15.9 | 24.1 | 16.2 | 35.5 | 31.9 | 38.3 |
| > 5 | 20.7 | 16.9 | 26.0 | 15.0 | 16.0 | 15.2 | 16.1 | 16.3 | 16.6 |
| P value | 0.002 | 0.564 | < 0.001 | 0.036 | 0.191 | 0.218 | 0.23 | 0.001 | < 0.001 |
Nodal stage (P = 0.020 in follow up before 2008, P = 0.014 in the overall study population), AJCC 2002 nodal stage (P = 0.011 in follow up before 2008, P = 0.001 in the overall study population), AJCC 2010 nodal stage (P = 0.039 in the overall study population) and the number of involved lymph nodes (P = 0.036 in follow up before 2008) were significantly associated with DMF survival (Table 4).
Age (P = 0.014 in the overall study population), vascular involvement (P < 0.001 in follow up before 2008 and in the overall study population), T stage (P = 0.04 in follow up before 2008 and P = 0.002 in the overall study population), nodal stage, AJCC 2002 nodal stage and AJCC 2010 nodal stage in follow up before 2008 and in the overall study population, P < 0.001 for each) and the number of involved lymph nodes (P = 0.001 in follow up before 2008 and P < 0.001 in the overall study population) were the significant correlates of overall survival (Table 4).
None of the variables determined to be significantly associated with LRF survival in the univariate analysis (vascular involvement and number of involved lymph nodes) was significant correlates of LRF survival in the multivariate analysis in patients followed before 2008. Multivariate analysis confirmed the association between nodal stage and LRF survival in the overall study population with 4.959 fold (P = 0.042) increase in mortality in patients with nodal stage N2 compared to patients with nodal stage N0, while no significant association was noted in terms of number of involved lymph nodes (Table 5).
| Local recurrence free survival | Distant metastasis free survival | Overall survival | ||||||||||||||||
| Before 2008 | 2008-2010 | Overall | Before 2008 | 2008-2010 | Overall | Before 2008 | 2008-2010 | Overall | ||||||||||
| Sig. | Exp(B) | Sig. | Exp(B) | Sig. | Exp(B) | Sig. | Exp(B) | Sig. | Exp(B) | Sig. | Exp(B) | Sig. | Exp(B) | Sig. | Exp(B) | Sig. | Exp(B) | |
| Age (yr) | ||||||||||||||||||
| < 50 vs > 50 | 0.748 | 1.191 | 0.387 | 1.468 | 0.122 | 1.742 | ||||||||||||
| Operaton type | ||||||||||||||||||
| Total vs subtotal | 0.118 | 0.284 | 0.147 | 0.426 | ||||||||||||||
| Vascular involvement | ||||||||||||||||||
| Yes vs No | 0.748 | 0.707 | 0.099 | 2.375 | 0.085 | 2.106 | ||||||||||||
| Perinueral involvement | ||||||||||||||||||
| Yes vs No | 0.656 | 0.820 | 0.326 | 0.707 | ||||||||||||||
| T stage | 0.772 | 0.318 | 0.781 | 0.762 | ||||||||||||||
| T1 | 0.938 | 10856.260 | 0.936 | 18774.638 | 0.995 | 0.995 | 0.967 | 0.967 | ||||||||||
| T2 | 0.934 | 21845.678 | 0.935 | 23091.062 | 0.674 | 1.425 | 0.651 | 1.447 | ||||||||||
| T3 | 0.933 | 24325.273 | 0.928 | 62667.340 | 0.969 | 0.962 | 0.734 | 1.369 | ||||||||||
| LN1 | ||||||||||||||||||
| < 15 vs > 16 | 0.458 | 2.278 | 0.722 | 0.863 | ||||||||||||||
| LN2 | ||||||||||||||||||
| < 10 vs > 11 | 0.073 | 0.146 | ||||||||||||||||
| Nodal stage (AJCC 2010) | 0.782 | 0.167 | 0.994 | 0.052 | 0.033 | 0.104 | ||||||||||||
| N1 | 0.255 | 3.526 | 0.217 | 2.748 | 0.754 | 1.196 | 0.831 | 1.118 | 0.404 | 1.624 | 0.162 | 2.184 | ||||||
| N2 | 0.232 | 3.853 | 0.0421 | 4.959 | 0.969 | 1.025 | 0.313 | 1.614 | 0.122 | 2.439 | 0.0091 | 4.056 | ||||||
| N3a | 0.452 | 3.407 | 0.786 | 1.380 | 0.814 | 1.266 | 0.014 | 3.218 | 0.0061 | 5.132 | 0.059 | 4.733 | ||||||
| N3b | 0.344 | 5.380 | 0.502 | 2.330 | 0.761 | 1.471 | 0.060 | 3.446 | 0.0271 | 5.263 | 0.098 | 4.292 | ||||||
| İnvolved lymph nodes (n) | ||||||||||||||||||
| ≤ 5 vs > 5 | 0.464 | 2.322 | 0.242 | 2.573 | 0.283 | 2.677 | 0.207 | 2.245 | 0.303 | 1.742 | 0.726 | 1.245 | ||||||
| Significance of the model | P = 0.053 | P = 0.218 | P = 0.017 | P = 0.153 | P = 0.196 | P = 0.054 | P = 0.001 | P = 0.151 | P < 0.0011 | |||||||||
None of the variables determined to be significantly associated with DMF survival in the univariate analysis (nodal stage and number of involved lymph nodes) was significant correlates of DMF in the multivariate analysis in patients followed before 2008 or in the overall study population (Table 5).
Except for 5.132 fold (P = 0.006), 5.263 fold (P = 0.027) and 4.056 fold (P = 0.009) increase in the mortality in patients with nodal stage N3a (before 2008), N3b (before 2008) and N2 (overall study population) when compared to patients with N0 stage, respectively, none of the variables significantly associated with overall survival in the univariate analysis was significant correlates of overall survival in the in the multivariate analysis in patients followed before 2008 and in the overall study population (Table 5).
The present retrospective single centre analysis of survival data (1998-2010) in patients with gastric carcinoma revealed median 31.9 mo of LRF survival, 24.1 mo of DMF survival and 31.9 mo of overall survival via postoperative adjuvant CRT during follow up from 1998 to 2010 with local recurrence rate of 13.7% in the overall study population. No significant difference was observed in median duration of LRF survival, DMF survival and overall survival with respect to treatment protocols (CRT vs CT), interval between surgery and radiotherapy and the type of radiotherapy (2-D vs 3-D), while FUFA (29.9 mo) and UFT (42.5 mo) based CT protocols and absence of positive surgical margin (27.7 mo) were associated with significantly longer median durations of LRF survival and overall survival, respectively. Multivariate analysis revealed higher nodal stage to be a significant determinant of LRF in the overall study population, while to predict overall survival both in patients followed up in 1998-2008 and in overall study population.
Regardless of the follow up period, adenocarcinoma type, location in antrum, poor differentiation, T3T4 and N1-N2 stage were the leading histopathological characteristics of the tumor as identified in most of patients. Patients followed up in 2008-2010 period were associated with significantly higher rate of signet ring cell type of carcinoma, vascular involvement, use of CRT and CEF based CT protocols as well as 3-D conformal technique in RT when compared to patients followed up before 2008, while the two groups of follow up were homogenous in terms of demographic and other clinicopathological characteristics.
Although the demonstration of the efficacy of postoperative CRT for locally advanced gastric cancer in randomized clinical trials provide a basis for the consideration of this therapy as the standard of care for resectable high-risk disease, local recurrence rates have been indicated to remain at 19% even after adjuvant CRT[2,4], which seems in line with the local recurrence rate (13.7%) demonstrated in our study population.
Although the efficacy of postoperative CT following complete resection has been associated with a significant survival benefit in some studies especially with fluoropyrimidine based regimens[2,3,14].
Nonetheless, preceding the landmark Intergroup Trial INT-0116 on the effect of surgery plus postoperative CRT on the survival of patients resected for adenocarcinoma of the stomach after a 10-year median follow-up[2], postoperative adjuvant CRT has consistently been associated with improved overall and relapse free survivals rates in comparison to patients without CRT in several clinical trials[2,15-17].
Past studies concerning direct comparison of CT plus radiotherapy with CT-only in patients with gastric cancer revealed significant improvement in disease free survival[12] and in median duration of relapse-free survival with 30 mo vs 19 mo[2] and 50 mo vs 36 mo)[18], while a significant increase (36 mo vs 27 mo)[2] as well as no significant improvement (58 mo vs 48 mo)[18] were noted for overall survival in CRT vs CT-only arm.
Accordingly, albeit not statistically significant, a tendency for higher rates for LRF survival (31.9 mo vs 25.9 mo), DMF survival (24.1 mo vs 20.6 mo) and overall survival (31.9 mo vs 27.1 mo) with postoperative CRT vs CT in our study population are in agreement with the survival benefit of CRT indicated in the past studies.
Indeed, due to variability of accepted standards for incorporation of postoperative CRT into the routine clinical practice in different countries, the ideal oral chemotherapeutic agent to be used in CRT protocol has not yet been defined[6].
Tried primarily in advanced stage gastric cancer as an alternative to FU, UFT was shown to be as effective as FU in postoperative therapy in the past studies[19,20]. Notably, type of CT protocol had significant influence on LRF survival but not on DMF survival and overall survival in our study population with significantly improved LRF survival rates obtained similarly in patients received UFT (42.5 mo) and FUFA (29.9 mo) based regimens. This finding seems in line with the previously emphasized survival benefit of fluoropyrimidine (FT) as well as FU[13,15] based regimens, while also supports that concurrent UFT with radiotherapy is an equally effective regimen in the postoperative treatment of gastric adenocarcinoma when compared to FUFA[6].
It should also be noted that restriction of the radiation dose to the intra-abdominal target volume which to 45-50 Gy due to adjacent dose-limiting organs in conventional RT has been suggested not be sufficient for disease control in patients with locally advanced gastric adenocarcinoma[4,21]. Nonetheless, use of escalated radiation doses with concurrent CT in an adjuvant setting has currently been considered as a strategy that deserves to be optimized and further evaluated in randomized clinical trials[4].
Extended interval between surgery and radiation has been considered to allow accelerate proliferation of cancer cells under stress and thus delivery of a larger dose early in the course of treatment has been suggested to further improve disease control of gastric cancer after surgical resection[4]. However, in our study population, the interval between surgery and radiotherapy initiation had no significant impact on survival times and similar values for overall survival was noted in patients who underwent radiotherapy within 4 mo (40.9 mo) vs after 4 mo (39.8 mo) of surgery.
Divided based on introduction of 3-D conformal technique in radiotherapy in 2008 at our clinic, the two consecutive periods of follow up (from 1998 to 2008 and from 2008 to 2010) in our study showed distinct median durations for LRF survival (37.8 mo vs 24.1 mo), DMF survival (11.7 mo vs 19.2 mo) and overall survival (31.9 mo vs 24.1 mo) with postoperative adjuvant CRT. However one must remain prudent when comparing these results, given that no significant difference was noted in median duration of survival with respect to treatment protocols (CRT vs CT) as well as type of radiotherapy (2-D vs 3D) along with higher proportion of patients under UFT therapy in the 1998-2008 group, and more importantly the remarkable difference between these groups in terms of duration of follow up (10 years vs 3 years). In this regard, longer term follow-up is needed to determine the actual treatment outcome and thereby the optimal therapy in our patients with gastric cancer.
Additionally, it should be emphasized that increasing evidence for a survival advantage from adjuvant therapies seems to enable postoperative CRT to become standard practice in patients with resectable gastric cancer only if treatment-related complications are minimized to ensure the maintenance of the survival advantage[2,15,17].
Lymph node metastasis was reported amongst the prognostic factors for gastric cancer in several studies[22-24]. In patients with gastric cancer, the 5-year survival rate N0, N1, N2, N3a and N3b after D2 lymph node dissection were reported to be 89.7%, 73.6%, 54.9%, 23.1% and 5.4%, respectively in a recent study[25], while positive lymph node and TNM stage were documented as independent prognostic factors for gastric cancer in a recent multivariate analysis[18]. Likewise, our findings indicated higher nodal stage as the common predictor of LRF survival in the overall population while of overall survival both in 1998-2008 group and in the overall study population
While higher nodal stage, T stage, and the number of involved lymph nodes were amongst the factors significantly associated with overall survival according to univariate analysis in our study population, these findings were not confirmed in the multivariate analysis. Larger scale studies with longer term follow up are needed to clarify prognostic determinants in patients with gastric carcinoma who received postoperative adjuvant CRT.
Certain limitations to this study should be considered. The major limitation seems to be the difference among study groups with respect to duration of follow up. Due to switching from 2-D to 3-D conformal technique in radiotherapy at our clinic in 2008, overall population was evaluated as divided into two consecutive periods of follow up including periods from 1998 to 2008 and between 2008 and 2010. However since data from patients in the first group are based on remarkably longer follow up of 10 years when compared to data from patients in the second group with 3 years of follow up, difference in survival times between two groups should be cautiously interpreted, given that no difference was noted in median duration of survival with respect to type of either post-adjuvant treatment protocol or the radiotherapy. Secondly, retrospective design seems to be another pitfall of our study which disabled to apply standard inclusion criteria and to enable patients to be prospectively randomized into treatment groups. Nevertheless, while based on a retrospective analysis of a single institution, our findings represent a solid ground for future larger scale prospective studies on comparison of different postoperative adjuvant treatment protocols in patients with gastric cancer in the longer term follow up.
In conclusion, based on identification of median 31.9 mo of LRF survival, 24.1 mo of DMF survival and 31.9 mo of overall survival via postoperative adjuvant CRT during follow up from 1998 to 2010 in our study population, our findings emphasize the likelihood of postoperative adjuvant CRT to have a survival benefit in patients with resectable gastric carcinoma. Use of FUFA and UFT based CT protocols and the absence of positive surgical margin in patients received CRT seems to be in favor of LRF survival and overall survival, respectively, while no significant difference was observed in duration of survival with respect to treatment protocols (CRT vs CT), interval between surgery and radiotherapy and the type of radiotherapy (2-D vs 3-D). Nodal stage was the independent prognostic factor for LRF and overall survival, while concluding the efficacy of post adjuvant CRT and exact determinants of survival in gastric cancer patients seem to depend on conduction of future prospective randomized trials on comparison of surgery only and postoperative CRT within a longer period of follow-up.
Implication of neoadjuvant or adjuvant therapy in patients with resectable gastric cancer mainly in the form of postoperative chemoradiotherapy (CRT) and perioperative chemotherapy (CT) has been considered by several studies in terms achievement of better therapeutic outcomes and shown to be associated with high-level evidence for improved survival in Western populations.
Despite increasing evidence available for a survival advantage from adjuvant therapies, adjuvant treatment strategies in patients with resectable gastric cancer still remains debated particularly in terms of favour of radiotherapy associated with CT, the adequacy of nodal dissection, the likelihood of CT related toxic effects and inconsistency of different therapeutic trials in terms of survival and relapse rates.
The present retrospective single centre analysis of survival data (1998-2010) in patients with gastric carcinoma revealed median 31.9 mo of local recurrence free (LRF) survival, 24.1 mo of distant metastasis free (DMF) survival and 31.9 mo of overall survival via postoperative adjuvant CRT during follow up from 1998 to 2010 with local recurrence rate of 13.7% in the overall study population. Albeit not statistically significant, a tendency for higher rates for LRF survival, DMF survival and overall survival with postoperative CRT vs CT in the study population are in agreement with the survival benefit of CRT indicated in the past studies. Extended interval between surgery and radiation has been considered to allow accelerate proliferation of cancer cells under stress and thus delivery of a larger dose early in the course of treatment has been suggested to further improve disease control of gastric cancer after surgical resection.
The findings emphasize the likelihood of postoperative adjuvant CRT to have a survival benefit in patients with resectable gastric carcinoma. Use of 5-fluorouracil and leucovorin-folinic acid and uracil/tegafur based CT protocols and the absence of positive surgical margin in patients received CRT seems to be in favor of LRF survival and overall survival, respectively, while no significant difference was observed in duration of survival with respect to treatment protocols (CRT vs CT), interval between surgery and radiotherapy and the type of radiotherapy (2-D vs 3-D) and nodal stage was the independent prognostic factor for LRF and overall survival.
Patients with gastric cancer show poor prognosis and cure rate remains dismal with 5-year survival rates of 8%-34% and locoregional recurrence of 40%-90% even after curative resection. The implication of neoadjuvant or adjuvant therapy in patients with resectable gastric cancer mainly in the form of postoperative CRT and perioperative CT has been considered by several studies in terms achievement of better therapeutic outcomes.
The authors performed retrospective single centre analysis of survival data (1998-2010) in patients with gastric carcinoma after curative resection. Theirs findings emphasize the likelihood of postoperative adjuvant CRT to have a survival benefit in patients with resectable gastric carcinoma. The authors concluded that prognosis of gastric cancer cases after curative resection with postoperative adjuvant chemoradiotherapy was better that that of cases without postoperative adjuvant.
P- Reviewer: Aoyagi K, Kabir A, Zhu YL S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88:921-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2465] [Cited by in RCA: 2431] [Article Influence: 101.3] [Reference Citation Analysis (0)] |
| 3. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4582] [Article Influence: 241.2] [Reference Citation Analysis (0)] |
| 4. | Zhang Q, Tey J, Peng L, Yang Z, Xiong F, Jiang R, Liu T, Fu S, Lu JJ. Adjuvant chemoradiotherapy with or without intraoperative radiotherapy for the treatment of resectable locally advanced gastric adenocarcinoma. Radiother Oncol. 2012;102:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1491] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 6. | Yoney A, Bati Y, Akboru H, Isikli L, Unsal M. A retrospective comparison of concurrent 5-fluorouracil or oral UFT in postoperative chemoradiation for gastric adenocarcinoma. Cancer Radiother. 2010;14:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Valentini V, Cellini F, Minsky BD, Mattiucci GC, Balducci M, D’Agostino G, D’Angelo E, Dinapoli N, Nicolotti N, Valentini C. Survival after radiotherapy in gastric cancer: systematic review and meta-analysis. Radiother Oncol. 2009;92:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Coburn NG, Guller U, Baxter NN, Kiss A, Ringash J, Swallow CJ, Law CH. Adjuvant therapy for resected gastric cancer--rapid, yet incomplete adoption following results of intergroup 0116 trial. Int J Radiat Oncol Biol Phys. 2008;70:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Brooks GA, Enzinger PC, Fuchs CS. Adjuvant therapy for gastric cancer: revisiting the past to clarify the future. J Clin Oncol. 2012;30:2297-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Michel P, Breysacher G, Mornex F, Seitz JF, Pere-Verge D, Martel-Lafay I, Faroux R, Chapet S, Sobhani I, Pezet D. Feasibility of preoperative and postoperative chemoradiotherapy in gastric adenocarcinoma. Two phase II studies done in parallel. Fédération Francophone de Cancérologie Digestive 0308. Eur J Cancer. 2014;50:1076-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, Gunderson LL, Goldman B, Martenson JA, Jessup JM. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 625] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 12. | Lee J, Lim do H, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 579] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 13. | Dahan L, Atlan D, Bouché O, Mitry E, Ries P, Artru P, Richard K, Lledo G, Nguyen T, Rougier P. Postoperative chemoradiotherapy after surgical resection of gastric adenocarcinoma: can LV5FU2 reduce the toxic effects of the MacDonald regimen? A report on 23 patients. Gastroenterol Clin Biol. 2005;29:11-15. [PubMed] |
| 14. | Cirera L, Balil A, Batiste-Alentorn E, Tusquets I, Cardona T, Arcusa A, Jolis L, Saigí E, Guasch I, Badia A. Randomized clinical trial of adjuvant mitomycin plus tegafur in patients with resected stage III gastric cancer. J Clin Oncol. 1999;17:3810-3815. [PubMed] |
| 15. | Kim S, Lim DH, Lee J, Kang WK, MacDonald JS, Park CH, Park SH, Lee SH, Kim K, Park JO. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;63:1279-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Park SH, Kim DY, Heo JS, Lim DH, Park CK, Lee KW, Choi SH, Sohn TS, Kim S, Noh JH. Postoperative chemoradiotherapy for gastric cancer. Ann Oncol. 2003;14:1373-1377. [PubMed] |
| 17. | Pemberton L, Coote J, Perry L, Khoo VS, Saunders MP. Adjuvant chemoradiotherapy for gastric carcinoma: dosimetric implications of conventional gastric bed irradiation and toxicity. Clin Oncol (R Coll Radiol). 2006;18:663-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Zhu WG, Xua DF, Pu J, Zong CD, Li T, Tao GZ, Ji FZ, Zhou XL, Han JH, Wang CS. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother Oncol. 2012;104:361-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Kim YH, Cheong SK, Lee JD, Park JS, Shin SW, Kim JS. Phase II trial of Oral UFT and leucovorin in advanced gastric carcinoma. Am J Clin Oncol. 1996;19:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Ravaud A, Borner M, Schellens JH, Geoffrois L, Schöffski BP, Kroon K, Wanders J, Hanauske AR, Fumoleau P. UFT and leucovorin in first-line chemotherapy for patients with metastatic gastric cancer. An Early Clinical Studies Group (ECSG)/European Organization for Research Treatment of Cancer (EORTC) phase II trial. Eur J Cancer. 2001;37:1642-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Withers HR, Peters LJ, Taylor JM. Dose-response relationship for radiation therapy of subclinical disease. Int J Radiat Oncol Biol Phys. 1995;31:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 157] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Wang W, Sun XW, Li CF, Lv L, Li YF, Chen YB, Xu DZ, Kesari R, Huang CY, Li W. Comparison of the 6th and 7th editions of the UICC TNM staging system for gastric cancer: results of a Chinese single-institution study of 1,503 patients. Ann Surg Oncol. 2011;18:1060-1067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Ahn HS, Lee HJ, Hahn S, Kim WH, Lee KU, Sano T, Edge SB, Yang HK. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2010;116:5592-5598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 24. | Qiu MZ, Wang ZQ, Zhang DS, Liu Q, Luo HY, Zhou ZW, Li YH, Jiang WQ, Xu RH. Comparison of 6th and 7th AJCC TNM staging classification for carcinoma of the stomach in China. Ann Surg Oncol. 2011;18:1869-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Chae S, Lee A, Lee JH. The effectiveness of the new (7th) UICC N classification in the prognosis evaluation of gastric cancer patients: a comparative study between the 5th/6th and 7th UICC N classification. Gastric Cancer. 2011;14:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |