INTRODUCTION
Esophageal benign lesions have a diverse spectrum of etiologies in terms of clinical course and underlying pathologic features. Benign esophageal tumors, while uncommon compared with esophageal carcinoma, can sometimes cause dysphagia but often have insignificant clinical outcomes. Endoscopic findings are essential for detection, diagnosis, staging, and treatment planning. Benign esophageal tumors are rare, with a prevalence ≤ 0.5%[1], while benign tumors represent 20% of esophageal neoplasms on autopsy[2]. Since many of these tumors are small and asymptomatic, few benign esophageal lesions attract clinical attention. Benign esophageal lesions could be detected more often with the widespread use of endoscopes, radiologic imaging[3], and increased awareness of the disease. Esophageal lesions can be classified in two different ways; histologically depending on the involved layer into epithelial or subepithelial lesions and endoscopically depending on endoscopic features such as flat, raised, or cystic lesions.
In this article, we review the endoscopic and pathological features of esophageal benign lesions. In all, 149 benign esophageal lesions in 2997 endoscopic examinations are retrospectively reviewed. We removed the esophageal epithelial lesions by biopsy or resected the subepithelial lesions by endoscopic mucosal resection or endoscopic submucosal dissection for histological analysis. In this article, we divide these lesions into epithelial or subepithelial lesions based on the final histological findings. The Institutional Review Board at Mackay Memorial Hospital approved this project.
EPITHELIAL LESIONS
Heterotopic gastric mucosa
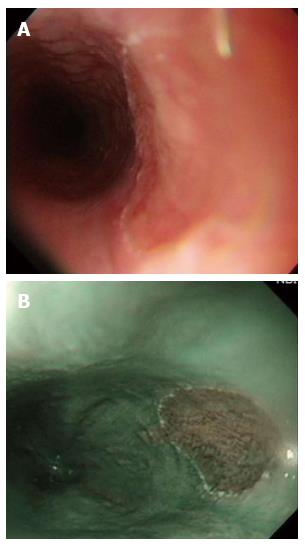
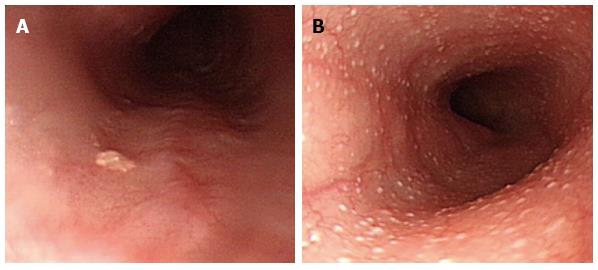
In all, 21 patients had histologically proven heterotopic gastric mucosa (HGM) (12 men, 9 women; mean age: 48 years, range: 25-69 years), which appears salmon-colored under conventional esophagogastroduodenoscopy (EGD) and is recognized as flat or slightly elevated (Figure 1A), while the iodine reaction is negative. Narrow band imaging facilitates mucosal surface evaluation by adjusting the reflected light to enhance the contrast between the esophageal mucosa and the HGM and may improve the diagnostic rate of HGM (Figure 1B). It is easily overlooked because it is typically located just below the upper esophageal sphincter.
Figure 1 Heterotopic gastric mucosa.
A: Heterotopic gastric mucosa appears salmon-colored under conventional endoscopy and is recognized as flat or slightly elevated; B: Narrow band imaging facilitates mucosal surface evaluation of heterotopic gastric mucosa by adjusting reflected light to enhance the contrast between the esophageal mucosa and the gastric mucosa and may improve the diagnosis of heterotopic gastric mucosa.
The prevalence of HGM is 0.1%-10%[4-6], and most patients have no symptoms[5,6]. Some patients present with pharyngeal globus sensation, heartburn, acid regurgitation, or dysphagia because HGM produces mucin and acid. Two types of HGM have been recognized: one with foveolar epithelium and fundic glands and the other with only foveolar epithelium[7]. The foveolar epithelium produces neutral mucins. The number of reported cases with neoplastic changes or malignant transformation is very low and these cases are considered extremely rare[8]. Management is generally required only if symptoms or complications develop.
Squamous cell papilloma
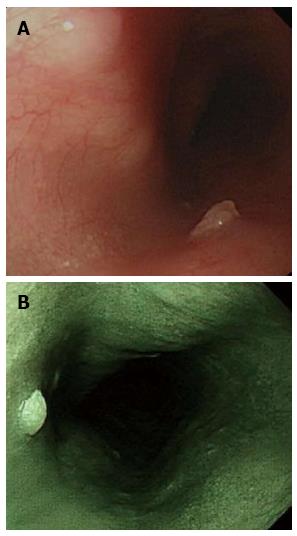
In all, 20 patients had histologically proven squamous cell papilloma (SCP) (3 men, 17 women; mean age: 49 years, range: 26-68 years), which is recognized as whitish-pink, wart-like exophytic projections on conventional endoscopy (Figure 2A) and is a rare benign esophageal lesion, with a prevalence of 0.01%-0.45%[9,10]. Narrow band imaging facilitates mucosal surface evaluation of squamous cell papilloma and shows that microvessels in the lesion are not dilated (Figure 2A). Most cases of SCP are solitary and asymptomatic[10,11]. The etiology of SCP is not fully understood, although there are two possible hypotheses: chronic mucosal irritation and infection with human papillomaviruses[12]. The hypotheses of inflammatory reactions such as gastroesophageal reflux disease were based on the high frequency of SCP occurring in the lower third of the esophagus. To our best knowledge, whether SCP is associated with human papillomavirus is controversial[13]. Papillomatosis in the proximal esophagus seems to favor involvement of the human papillomavirus[14].
Figure 2 Squamous cell papilloma.
A: Squamous cell papilloma is recognized as whitish-pink, wart-like exophytic projections on conventional endoscopy; B: Narrow band imaging facilitates mucosal surface evaluation of squamous cell papilloma and shows that microvessels in the lesion are not dilated.
In the majority of the cases, SCP of the esophagus can be removed using endoscopic biopsy forceps because most are only a few millimeters in size. Larger papillomas can be removed using endoscopic mucosectomy. We suggest removing these lesions endoscopically because, while rare, these lesions have malignant potential.
Hyperplastic polyp
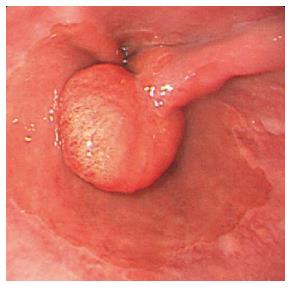
In all, 18 patients had histologically proved hyperplasia polyp (HP) (12 men, 6 women; mean age: 36 years, range: 18-69 years). In our patients, HPs were most common in the region of the esophago cardiac junction (67%), followed by the distal esophagus (27%) and mid-esophagus (6%). HP appears as a polypoid lesion on edematous inflamed gastric folds at the gastroesophageal junction (Figure 3). HPs of the esophagus and esophagogastric junction region are characterized by the presence of mixed inflammatory infiltrates with plasma cells, eosinophils, fibroblasts, and inflamed stroma. HPs are associated with concurrent erosive esophagitis in the majority of cases, but other potential etiologies including medication-induced pill esophagitis, infection, and previous anastomosis or polypectomy have been reported[15]. These results suggest that the pathogenesis of HP is a mucosal regenerative response to surrounding mucosal injury[16].
Figure 3 Hyperplasia polyp.
Hyperplasia polyp occurs as a polypoid lesion and is located on edematous inflamed gastric folds at the gastroesophageal junction.
Treatment for HP is similar to that for gastroesophageal reflux. In our experience, HP regresses after anti-acid therapy such as proton pump inhibitors. Careful clinical history and biopsy of the non-polypoid mucosa are essential for determining the clinicopathological context in which the HP developed. When HP is found with Barrett’s esophagus, endoscopic mucosal resection is recommended[17].
Xanthoma
We reported on esophageal xanthomas localized in the lower esophagus in a 62-year-old man. EGD revealed some well-defined, fern-like, and yellowish lesions scattered over the middle and lower esophagus (Figure 4).
Figure 4 Xanthoma.
Xanthomas are endoscopically recognized as elevated, granular, yellowish, fern-like lesions and scattered on a normal mucosal surface.
In terms of endoscopic findings, xanthoma is most commonly solitary, 2-10 mm across, and located in the lower esophagus, but cases of multifocal lesions have been reported[18,19]. Microscopically, they consist of fat accumulation in foamy histiocytes beneath the squamous epithelium[20]. One study showed the most common location of ectopic xanthoma in the gastrointestinal tract was the stomach (76%), followed by the esophagus (12%) and duodenum (12%)[21]. The etiology of esophageal xanthoma remains unknown, but one study theorized that these xanthomas were derived from focal mucosal damage, and that lipids derived from broken down cell membranes are captured by interstitial histiocytes[13]. This may explain why they occur less frequently in the esophagus than stomach because the esophageal mucosa can better tolerate mucosal injury[21].
Xanthoma must be distinguished on endoscopy from other yellowish lesions such as carcinoid tumor, granular cell tumors, and ectopic sebaceous glands (ESGs).
ESGs
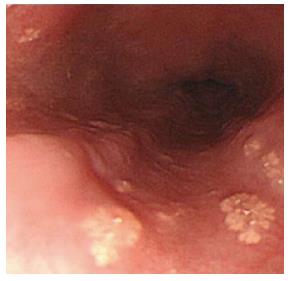
In all, three patients (one man and two women; mean age: 52 years, range: 45-69 years) had histologically proven ESGs within the esophagus. EGD revealed variable numbers from single (Figure 5A) to > 100 (Figure 5B) yellowish plaques measuring 1-2 mm in diameter in the middle and lower esophagus.
Figure 5 Ectopic sebaceous gland.
A: The number of ectopic sebaceous gland is variable from single to B: more than one hundred yellowish plaques measuring 1 to 2 mm in the esophagus.
ESGs have been found in various tissues, such as the lips and mouth, external genitalia, parotid glands, palms, soles, and various organs[22]. Typically, the mean age of affected patients is approximately 50 years, and the condition has equal gender distribution[23]. The numbers of ESGs in the esophagus varied from single to > 100, while their size was 1-20 mm (the majority were < 0.5 cm)[23,24]. The ESGs are most likely the result of a metaplastic process rather than a congenital anomaly because the ESGs are derived from endodermal tissue unlike the sebaceous glands, which are derived from ectodermal tissue[22,25].
The apparent low incidence of this condition may be because of the lack of obvious clinical signs and symptoms. Most cases have been discovered incidentally by endoscopy during a referral for a gastrointestinal tract examination. Most cases have no significant overall changes in ectopic sebaceous gland number, size, or shape during follow-up[26]. We suggest that ESGs do not need further treatment.
Glycogenic acanthosis
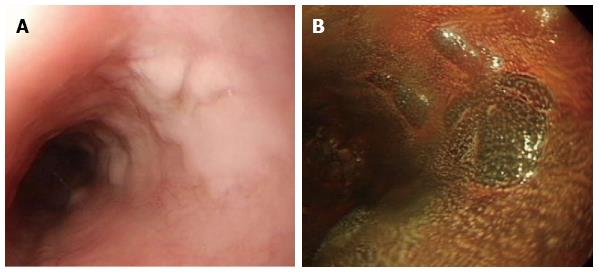
Glycogenic acanthosis was demonstrated as nodules involving otherwise normal esophageal mucosa in 66 patients (42 men and 24 women; mean age: 52 years, range: 45-79 years). Glycogenic acanthosis is defined as nodules involving otherwise normal esophageal mucosa. This is endoscopically recognized as slightly elevated areas on conventional endoscopy (Figure 6A) and slightly elevated iodine-positive areas on chromoscopy (Figure 6B). These lesions most commonly appear as multiple, uniformly sized, and oval or round elevations < 1 cm in diameter[27]. Biopsies after periodic acid-Schiff staining demonstrate that the nodules that represent glycogenic acanthosis are combinations of cellular hyperplasia and increased cellular glycogen[27]. Although glycogenic acanthosis was thought to be related to gastroesophageal reflux, antireflux therapy improved symptoms but failed to eradicate glycogenic acanthosis lesions[27,28].
Figure 6 Glycogenic acanthosis.
A: Esophagogastroduodenoscopy reveals multiple, uniformly sized, oval or round glycogenic acanthosis usually < 1 cm, involving otherwise normal esophageal mucosa; B: In chromoscopy with iodine spray, glycogenic acanthosis is recognized as slightly elevated iodine-positive, brownish areas.
SUBEPITHELIAL LESIONS
Leiomyoma
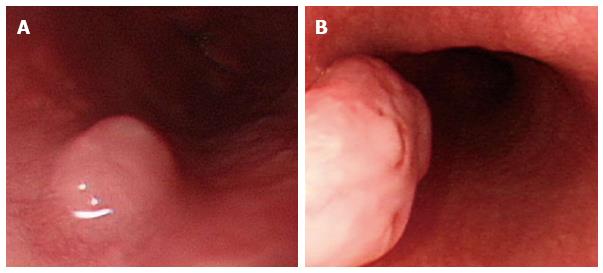
Leiomyoma was observed in three patients (3 men; mean age: 37 year, range: 25-43 years). Leiomyomas were located at the middle esophagus in 1 case and at the distal esophagus in 2 cases. The mean size of leiomyoma is 1.26 ± 0.4 cm. It commonly arises from the muscularis propria layer of the esophagus and presents as a submucosal tumor (Figure 7A). In rare circumstances, those cases arising from the muscularis mucosae can present as polypoid intraluminal tumors (Figure 7B).
Figure 7 Leiomyoma.
A: Leiomyoma commonly arises from the muscularis propria layer of the esophagus and presents as submucosal tumor; B: Leiomyoma arising from the muscularis mucosae can present as a polypoid intraluminal tumor.
Leiomyomas are the most common benign esophageal neoplasm, accounting for roughly two-thirds of all benign tumors of this organ[29]. Because they arise from smooth muscle cells, they are located mainly in the middle and distal thirds of the esophagus but are uncommon in the upper third of the esophagus, where the muscular layer is predominately skeletal in origin.
Most patients are asymptomatic, but dysphagia and pain may develop depending on lesion size and encroachment degree into the esophageal lumen. Treatment options include endoscopic enucleation[30], submucosal tunneling endoscopic resection[31], and surgical enucleation[32] or observation. Esophageal leiomyomas have a benign clinical course and typically do not recur after surgery.
Granular cell tumor
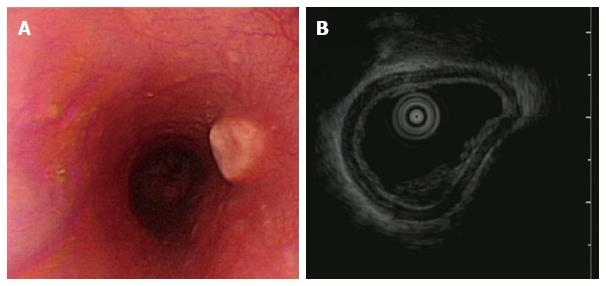
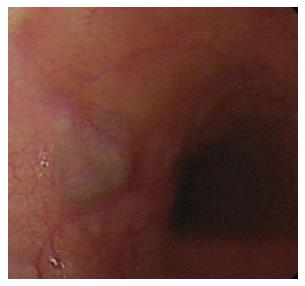
Granular cell tumor was observed in one 39-year-old health woman who presented with acid regurgitation and discomfort sensation when swallowing. EGD revealed one 12 mm × 15 mm firm, slightly elevated, whitish-to-yellow, and smooth nodular tumor covered by an intact epithelium (Figure 8A). Endoscopic ultrasonography showed one homogenous hypo-echogenic tumor extending from the muscularis mucosa layer (Figure 8B). The muscularis propria was not involved. Endoscopic submucosal dissection was performed.
Figure 8 Granular cell tumor.
A: Granular cell tumor is endoscopically recognized as a firm, yellowish subepithelial tumor covered with the normal mucosa in the esophagus; B: Endoscopic ultrasonography of granular cell tumor shows a homogenous hypo-echogenic tumor extending from the muscularis mucosa layer, and the musculais propria is not involved.
Granular cell tumors are the secondary common cause of non-epithelial tumors in the esophagus. Most of the patients with esophageal granular cell tumor are asymptomatic[33], but some complain of mild dysphagia or retrosternal discomfort. Although most granular cell tumors have a clinically indolent course, it is estimated that 1%-3% are malignant with a 5-year survival rate < 35%[34]. Microscopically, granular cell tumors are composed of nests of ovoid or polygonal cells separated by collagen bundles. The tumor has malignant potential in the presence of necrosis, increased mitotic count, vesicular nuclei with large nucleoi, and high Ki67 index[35].
Resection is the main treatment for granular cell tumors. Endoscopic mucosal resection and endoscopic submucosal dissection were introduced and were considered the therapy of choice for tumors within the subepithelial layer or submucosa separated from the muscularis propria.
Hemangioma (venous bleb)
Esophageal hemangiomas were observed in 13 patients (4 men, 9 women; mean age: 67 years, range: 45-82 years). On endoscopy, they appear cystic and bluish-red and can be pressed with biopsy forceps (Figure 9). The prevalence of esophageal hemangiomas in the general population was 0.04% based upon the findings of an autopsy series[36]. The majority of these hemangiomas are cavernous; however, capillary lesions have been described. Although usually solitary, multiple lesions can be seen in congenital blue rubber bleb nevus syndrome[37]. Esophageal hemangiomas are usually found incidentally. When symptomatic, they are most often associated with bleeding and dysphagia. In such cases, surgical resection has been performed, but endoscopic resection has also been accomplished safely[38]. Although esophageal hemangiomas are uncommon, careful consideration during endoscopy is required to avoid the misdiagnosis of varices[39].
Figure 9 Hemangioma.
On endoscopy, esophageal hemangioma appears cystic and bluish-red and can be pressed with biopsy forceps.
Dysphagia aortica
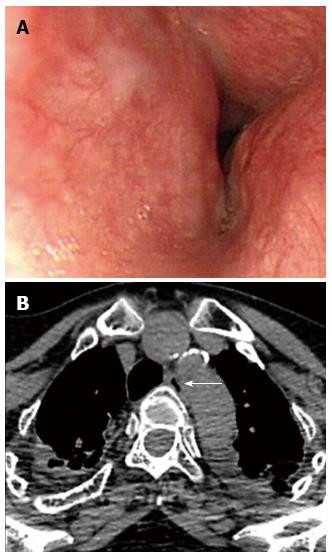
In dysphagia aortica was observed in three patients (1 man, 2 women; mean age: 68 years, range: 63-78 years). They visited our clinics with postprandial abdominal fullness and progressive dysphagia to solid meals. The esophagus begins on the right side of the thoracic aorta and then, descends and crosses the aorta anteriorly across its lower third. EGD reveals external compression of the middle-to-lower esophagus by the tortuous aorta with pulsations from the great vessels (Figure 10A).
Figure 10 Dysphagia aortica.
A: Esophagogastroduodenoscopy reveals a pulsatile extrinsic compression at about 25 cm from the incisor; B: The chest computed tomography showed aortic arch and descending aorta tortuosity with compression into adjacent esophagus. The arrow indicates the esophagus.
Dysphagia aortica is a rare etiology of dysphagia resulting from the extrinsic compression of the esophagus by a thoracic aortic aneurysm or the tortuosity and elongation of the thoracic aorta (Figure 10B). The clinical findings resemble those of esophageal malignancy or esophageal motility disorders. Esophageal compression by a vascular structure is a common cause of dysphagia[40]. Non-aneurysmatic aortic dysphagia is usually observed in the elderly, especially in hypertensive women with enlarged heart and kyphosis[41,42].
The treatment of dysphagia aortica depends on symptom severity. Symptoms of mild dysphagia often improve with diet modifications (e.g., by avoiding lying down immediately after taking drugs or food, eating small but frequent meals, and chewing well) and treatment of the coexistent disease, such as heart failure or hypertension. Some patients with more severe symptoms may respond to surgery[43,44].
Dysphagia aortica should be considered in the differential diagnosis of dysphagia, especially in the growing elderly population with underlying cardiovascular disease or hypertension.
CONCLUSION
Benign esophageal lesions have a lower detection rate due to the fact that most patients are asymptomatic. But it could be easily found with the widespread use of endoscopes and the increasing awareness of this disease. The majority of these benign tumors are asymptomatic, and diagnoses are often made incidentally during investigations for other complaints or symptoms. Although biopsy or excision is required for a definitive diagnosis, understanding the endoscopic appearances provides essential help for differential diagnosis. We suggest endoscopic resection of all granular cell tumors and squamous papillomas because, while rare, these lesions have malignant potential. Hyperplastic polyps could regress after anti-acid therapy such as proton pump inhibitors. We suggest that ectopic sebaceous glands and xanthoma do not need further treatment. Esophageal hemangiomas are uncommon, and careful consideration during endoscopy is required to avoid the misdiagnosis of varices. Dysphagia aortica should be considered in the differential diagnosis of dysphagia in the elderly.