Copyright
©The Author(s) 2015.
World J Gastroenterol. Oct 21, 2015; 21(39): 11127-11140
Published online Oct 21, 2015. doi: 10.3748/wjg.v21.i39.11127
Published online Oct 21, 2015. doi: 10.3748/wjg.v21.i39.11127
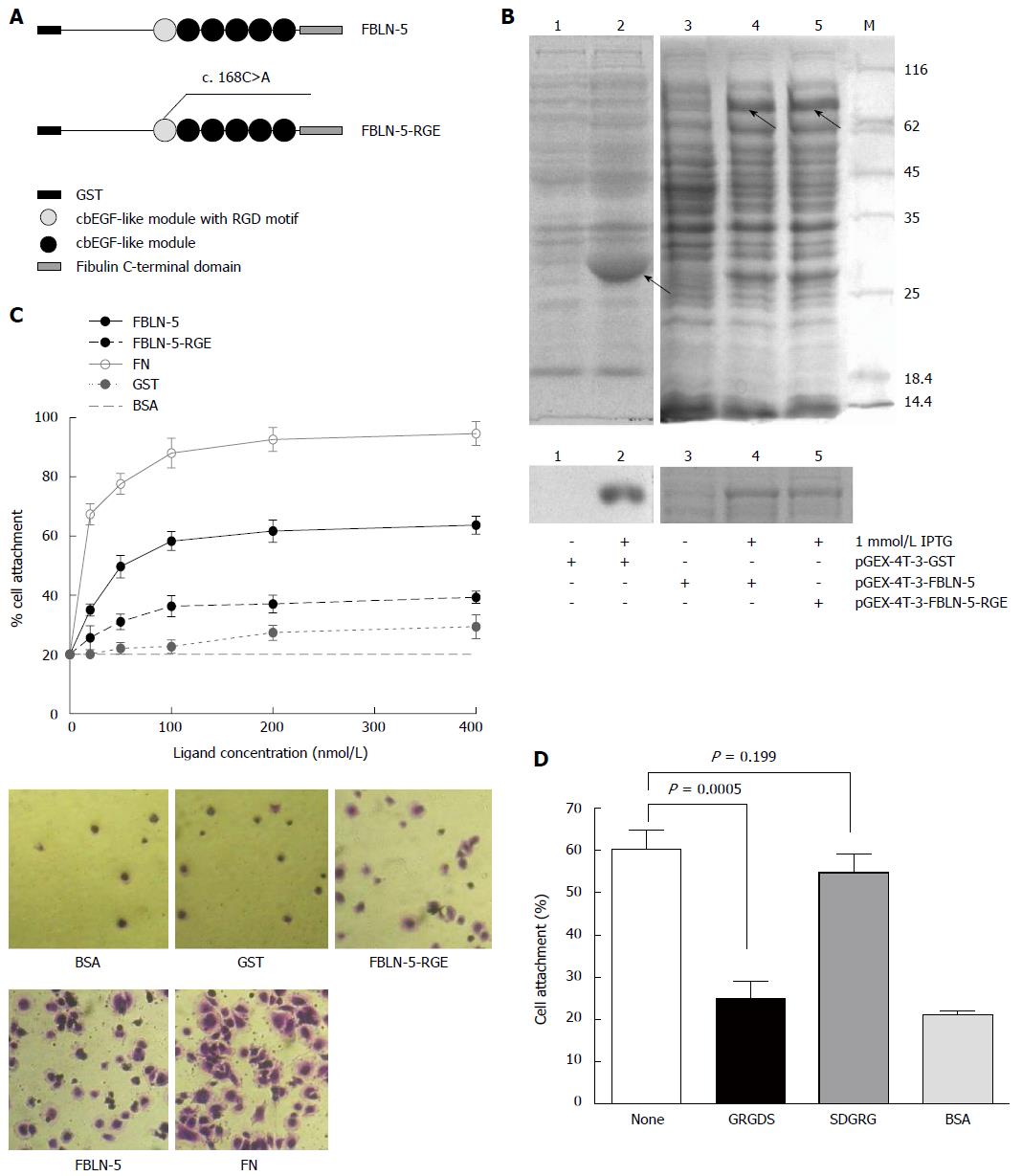
Figure 3 Characterization of recombinant human fibulin-5 and the attachment to hepatocellular carcinoma-LM3 cells.
A: Domain organization of recombinant fusion protein of GST-FBLN-5 and GST-FBLN-5-RGE (cbEGF-like module, calcium binding EGF-like module); B: SDS/PAGE analysis of glutathionesepharose affinity-purified GST, recombinant full-length FBLN-5, and FBLN-5-RGE under non-reducing conditions. M indicates molecular-mass markers (sizes in kDa). IPTG (1 mmol/L) significantly induced the expression of recombinant proteins (upper), which were purified with glutathionesepharose beads later (bottom); C: Attachment of HCC-LM3 cells on GST (black dotted line), FBLN-5 (black solid line), FBLN-5-RGE (black dashed line), and human plasma FN (grey solid line). Background cell adhesion on BSA-coated wells is also shown as grey dashed lines (upper). A high percentage of HCC-LM3 cells showed spreading on full-length FBLN-5 and on FN at 200 nmol/L, but many fewer cells spread on FBLN-5-RGE, GST and BSA were visible (bottom). Results are mean ± SD of three experiments; D: Inhibition of HCC-LM3 attachment to 200 nmol/L FBLN-5 by synthetic GRGDS peptide (500 μg/mL). Synthetic SDGRG peptide (500 μg/mL) was used as a negative control. None indicates HCC-LM3 attachment to 200 nm FBLN-5 in the absence of peptide. BSA indicates HCC-LM3 attachment to BSA-blocked wells. Pre-incubation of HCC-LM3 with the synthetic peptide GRGDS significantly blocked adhesion to FBLN-5 (P < 0.001, compared with untreated HCC-LM3 on FBLN-5). Pre-incubation with the control peptide SDGRG showed no inhibition of cell attachment (P = 0.199, compared with untreated cells on FBLN-5).
-
Citation: Tang JC, Liu JH, Liu XL, Liang X, Cai XJ. Effect of fibulin-5 on adhesion, migration and invasion of hepatocellular carcinoma cells
via an integrin-dependent mechanism. World J Gastroenterol 2015; 21(39): 11127-11140 - URL: https://www.wjgnet.com/1007-9327/full/v21/i39/11127.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i39.11127