Copyright
©The Author(s) 2015.
World J Gastroenterol. Oct 21, 2015; 21(39): 10936-10947
Published online Oct 21, 2015. doi: 10.3748/wjg.v21.i39.10936
Published online Oct 21, 2015. doi: 10.3748/wjg.v21.i39.10936
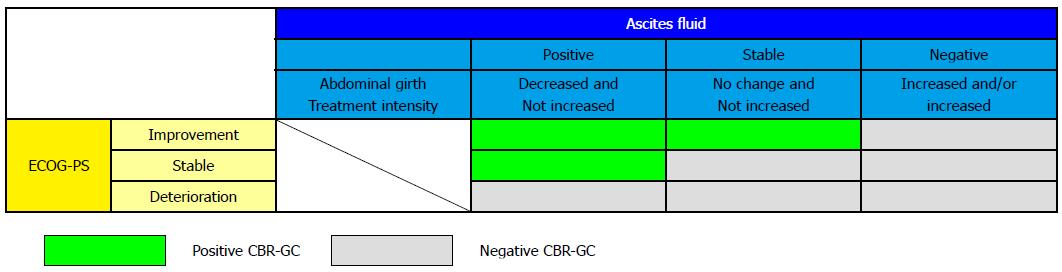
Figure 2 Clinical benefit response in gastric cancer.
CBR-GC is defined by the ascites response to treatment (horizontal axis) and ECOG-PS (vertical axis). Response of ascites is judged by a combination of abdominal girth and treatment intensity. CBR-GC: Clinical benefit response in gastric cancer; ECOG-PS: Eastern Cooperative Oncology Group performance status.
- Citation: Maeda H, Kobayashi M, Sakamoto J. Evaluation and treatment of malignant ascites secondary to gastric cancer. World J Gastroenterol 2015; 21(39): 10936-10947
- URL: https://www.wjgnet.com/1007-9327/full/v21/i39/10936.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i39.10936