Copyright
©The Author(s) 2015.
World J Gastroenterol. Oct 14, 2015; 21(38): 10874-10882
Published online Oct 14, 2015. doi: 10.3748/wjg.v21.i38.10874
Published online Oct 14, 2015. doi: 10.3748/wjg.v21.i38.10874
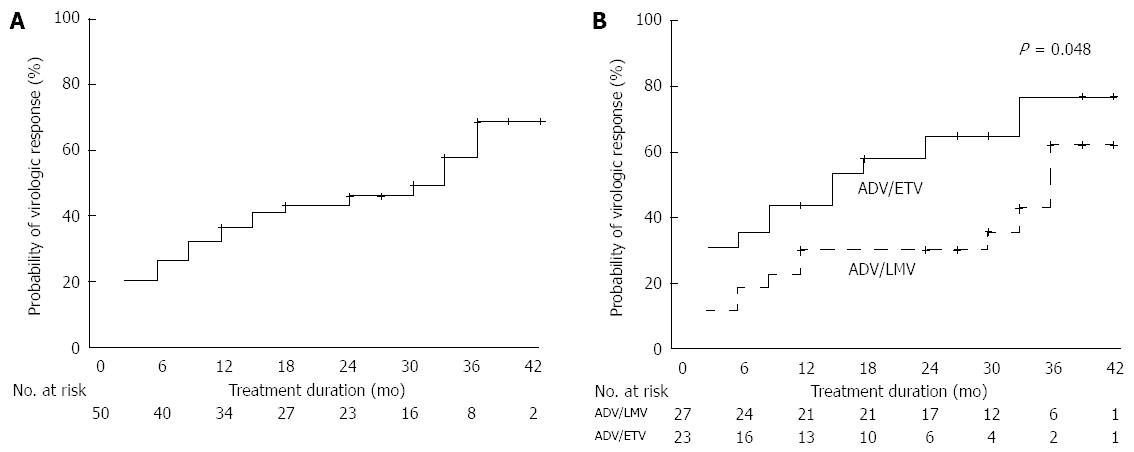
Figure 2 Virologic responses according to type of treatments up to 36 mo.
A: Overall cumulative virologic response rates at 6, 12, 24, and 36 mo; B: Cumulative virologic response rates in the adefovir plus entecavir (ADV/ETV) combination group and in the adefovir plus lamivudine (ADV/LMV) combination group (P = 0.048).
- Citation: Kim HS, Yim HJ, Jang MK, Park JW, Suh SJ, Seo YS, Kim JH, Kim BH, Park SJ, Lee SH, Kim SG, Kim YS, Lee JI, Lee JW, Kim IH, Kim TY, Kim JW, Jeong SH, Jung YK, Park H, Group SGHOBOARS. Management of entecavir-resistant chronic hepatitis B with adefovir-based combination therapies. World J Gastroenterol 2015; 21(38): 10874-10882
- URL: https://www.wjgnet.com/1007-9327/full/v21/i38/10874.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i38.10874