Published online Sep 21, 2015. doi: 10.3748/wjg.v21.i35.10200
Peer-review started: March 31, 2015
First decision: May 18, 2015
Revised: May 29, 2015
Accepted: July 18, 2015
Article in press: July 18, 2015
Published online: September 21, 2015
AIM: To evaluate the reliability and accuracy of three-dimensional (3D) reconstruction for liver resection in patients with hepatic alveolar echinococcosis (HAE).
METHODS: One-hundred and six consecutive patients with HAE underwent hepatectomy at our hospital between May 2011 and January 2015. Fifty-nine patients underwent preoperative 3D reconstruction and “virtual” 3D liver resection before surgery (Group A). Another 47 patients used conventional imaging methods for preoperative assessment (Group B). Outcomes of hepatectomy were compared between the two groups.
RESULTS: There was no significant difference in preoperative data between the two groups. Compared with patients in Group B, those in Group A had a significantly shorter operation time (227.1 ± 51.4 vs 304.6 ± 88.1 min; P < 0.05), less intraoperative blood loss (308.1 ± 135.4 vs 458.1 ± 175.4 mL; P < 0.05), and lower requirement for intraoperative blood transfusion (186.4 ± 169.6 vs 289.4 ± 199.2 mL; P < 0.05). Estimated resection liver volumes in both groups had good correlation with actual graft weight (Group A: r = 0.978; Group B: r = 0.960). There was a significant higher serum level of albumin in Group A (26.3 ± 5.9 vs 22.6 ± 4.3 g/L, P < 0.05). Other postoperative laboratory parameters (serum levels of aminotransferase and bilirubin; prothrombin time) and duration of postoperative hospital stay were similar. Sixteen complications occurred in Group A and 19 in Group B. All patients were followed for 3-46 (mean, 17.3) mo. There was no recurrence of lesions in Group A, but two recurrences in Group B. There were three deaths: two from cerebrovascular accident, and one from car accident.
CONCLUSION: 3D reconstruction provides comprehensive and precise anatomical information for the liver. It also improves the chance of success and reduces the risk of hepatectomy in HAE.
Core tip: With the rapid development of digital medicine, three-dimensional (3D) reconstruction software has become a new tool in surgery, and it is a quantitative imaging analysis system that provides real-time interactive tools for presurgical evaluation and planning. We compared the clinical results of hepatectomy for hepatic alveolar echinococcosis based on 3D software with traditional assessment. We found that liver resection based on 3D reconstruction was more effective in the diagnosis and treatment of HAE than techniques without 3D reconstruction. Such 3D reconstruction software for preoperative evaluation and surgical planning could increase the chance of success of surgery and reduce operative risk.
- Citation: He YB, Bai L, Aji T, Jiang Y, Zhao JM, Zhang JH, Shao YM, Liu WY, Wen H. Application of 3D reconstruction for surgical treatment of hepatic alveolar echinococcosis. World J Gastroenterol 2015; 21(35): 10200-10207
- URL: https://www.wjgnet.com/1007-9327/full/v21/i35/10200.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i35.10200
Human hepatic alveolar echinococcosis (HAE) is caused by the larval stage of Echinococcus multilocularis[1]. HAE is among the most dangerous zoonoses and has a high prevalence in rural areas of Western China[2,3]. HAE usually exhibits cancerous growth, and is referred to as “parasitic liver cancer”. The progress of HAE is slow (can be ≤ 10 years). Radical hepatic resection at early and middle stages of the disease can result in satisfactory outcomes, but recurrence is high[4]. In the late stages of HAE, radical resection usually cannot be undertaken due to serious violations of the conduits inside and outside of the liver by the lesion[5]. The lesion can metastasize, in which case the risk of mortality is high, leaving liver transplantation or palliative drug treatment as the only options[6]. Therefore, accurate anatomical evaluation and precise surgical management are crucial for HAE treatment.
In recent years, 3D reconstruction systems based on data from conventional imaging have become important tools for preoperative evaluation and planning for liver surgeons[7]. Several clinical studies have shown that, for complex space-occupying hepatic lesions, application of preoperative 3D reconstruction technology can improve the success and feasibility of procedures, and reduce the prevalence of procedure-related complications[8].
In the present study, conventional computed tomography or a 3D reconstruction planning system was used for preoperative evaluation and surgical planning in patients with HAE. By comparing outcomes and preoperative assessment methods, the value of 3D reconstruction systems for the surgical treatment of HAE was evaluated.
The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (Xinjiang, China). All patients were informed of the risk and complications of surgery and provided written informed consent. All surgical procedures were carried out by the authors.
One-hundred and six consecutive patients with HAE underwent hepatectomy at the First Affiliated Hospital of Xinjiang Medical University between May 2011 and January 2015. Clinical manifestations included varying degrees of upper-right abdominal pain and jaundice. Metastases to distant organs were not detected. Contraindications to surgery (e.g., abnormalities in cardiac or pulmonary functions) were not identified. Among 106 patients, 59 underwent liver resection based on 3D reconstruction (Group A) and the remaining cases underwent hepatic resection without 3D reconstruction (Group B). Each patient decided whether to undergo treatment using 3D reconstruction. Preoperative clinical data are listed in Table 1.
| Variable | Group A(n = 59) | Group B(n = 47) | P value |
| Age, yr, mean ± SD | 41.4 ± 13.1 | 42.5 ± 13.2 | 0.968 |
| Sex, male/female | 32/27 | 24/23 | 0.448 |
| Location of lesion, n (%) | |||
| Right lobe | 34 (57.6) | 30 (63.8) | 0.327 |
| Left lobe | 12 (20.3) | 9 (19.1) | 0.350 |
| Bilateral lobes | 13 (22.0) | 8 (17.0) | 0.347 |
| AST, U/L, mean ± SD | 37.6 ± 23.9 | 40.2 ± 28.9 | 0.362 |
| ALT, U/L, mean ± SD | 33.7 ± 30.6 | 33.2 ± 29.4 | 0.545 |
| ALB, g/L, mean ± SD | 41.5 ± 5.2 | 40.5 ± 5.9 | 0.573 |
| TBIL, mmol/L, mean ± SD | 25.3 ± 13.6 | 27.5 ± 18.9 | 0.182 |
| DBIL, mmol/L, mean ± SD | 14.2 ± 10.5 | 12.2 ± 13.2 | 0.325 |
| PT, s, mean ± SD | 13.1 ± 2.1 | 13.0 ± 2.6 | 0.655 |
Computed tomography (CT) images were obtained using a 64-slice Spiral CT system (Light speed VCT; GE Healthcare, Piscataway, NJ, United States). Slice thickness was 1.25 mm. Non-enhanced and three-phase contrast-enhanced scans (arterial, portal venous, and delayed phases) were taken. CT angiography (CTA) reconstruction was carried out after imaging. CT data of all patients were stored in DICOM format. Liver volume of 47 patients in Group B was measured using an image-processing workstation (GE Healthcare), and reviewed by a radiologist and a surgeon. Images of the portal venous phase with the most pronounced liver parenchyma were selected. Liver morphology was recorded for every four slices from the superior aspect of the liver. Area of each slice was measured, and resection planes designed by a surgeon. Resection liver volumes were calculated using the computing function of the workstation.
CT data in DICOM format for all 59 patients of Group A were imported into 3D reconstruction software (IQQA-Liver; EDDA Technology, Princeton, NJ, United States). This software recognizes the liver and reconstructs the spatial structure of blood vessels automatically, and that information is compared with the 2D CT image by image fusion. The small number of differences between the two reconstructed structures was reconciled manually. After reconstruction, data were saved as still images or dynamic screens. Real-time data (including cross-sectional diameters of each blood vessel in all patients) were determined by measurement tools in IQQA-Liver. Positional relationships between lesions and the hepatic vein, portal vein, and bile duct were defined. Involvement of the first and second hepatic portals, as well as the retrohepatic inferior vena cava, was analyzed. Virtual surgery was carried out on the reconstructed 3D model. Resection and remaining liver volumes were calculated in real-time to evaluate the feasibility of the proposed surgical strategy. The treatment plan was finalized after optimization of individualized virtual surgery. Work was completed in coordination with a technologist specializing in 2D CT and a surgeon.
Data were analyzed using SPSS version 19.0 (IBM, Armonk, NY, United States). Student’s t-test, χ2 test and Fisher’s exact test were used to compare data from patients in Groups A and B before, during and after surgery. Correlation between remaining liver volumes estimated preoperatively and those measured after surgery were analyzed by Pearson’s correlation test. P < 0.05 was considered significant.
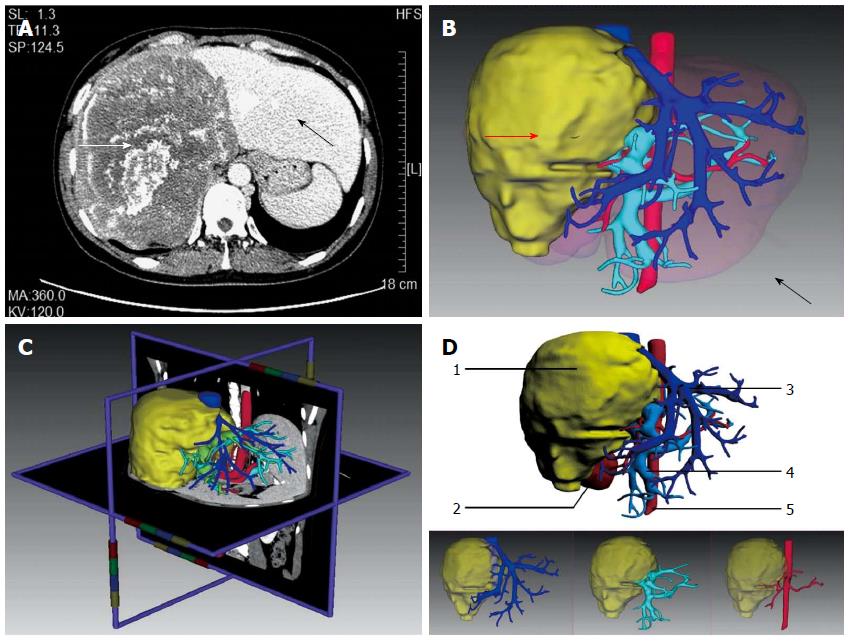
All 106 patients were confirmed to have HAE by CT (Figure 1A). 3D reconstruction of the liver clearly illustrated the positional relationship between the liver, lesions, and hepatic blood vessels in Group A (Figure 1B). Lesion area needed to be defined manually due to blurred edges in seven of 59 cases. Individualized reconstruction and preoperative evaluation were completed automatically by IQQA-Liver in the remaining cases.
Images (2D and 3D) were compared using the verification tools of the software, which revealed that the anatomical relationship among lesions and hepatic blood vessels in 3D reconstruction was consistent with that of the 2D images (Figure 1C). As displayed in the 3D reconstruction image, for the 59 patients in Group A, the first and second hepatic portals were invaded in different degrees. The inferior vena cava of 13 patients was surrounded (≤ 180°) by liver lesions (Figure 1D). Vessel invasion is shown in Table 2. The liver was divided into several hepatic parenchymal regions or sub-regions perfused by major blood vessels (including the hepatic and portal veins) to illustrate the spatial morphology of each segment of the liver.
| Vessels invaded | Group A (n = 59) | Group B (n = 47) | P value |
| HA | |||
| RHA | 30 (50.8) | 26 (55.3) | 0.397 |
| LHA | 9 (15.3) | 8 (17.0) | 0.505 |
| LHA and RHA | 20 (33.9) | 13 (27.7) | 0.317 |
| HV | |||
| RHV | 22 (37.3) | 19 (40.4) | 0.448 |
| LHV | 5 (8.5) | 8 (17.0) | 0.249 |
| RHV and MHV | 6 (10.2) | 7 (14.9) | 0.329 |
| LHV and MHV | 17 (28.8) | 13 (27.7) | 0.536 |
| PV | |||
| RPV | 31 (52.5) | 22 (46.8) | 0.348 |
| LPV | 11 (18.6) | 11 (23.4) | 0.358 |
| LPV and RPV | 17 (28.8) | 14 (29.8) | 0.541 |
| IVC | 13 (22.0) | 9 (19.1) | 0.453 |
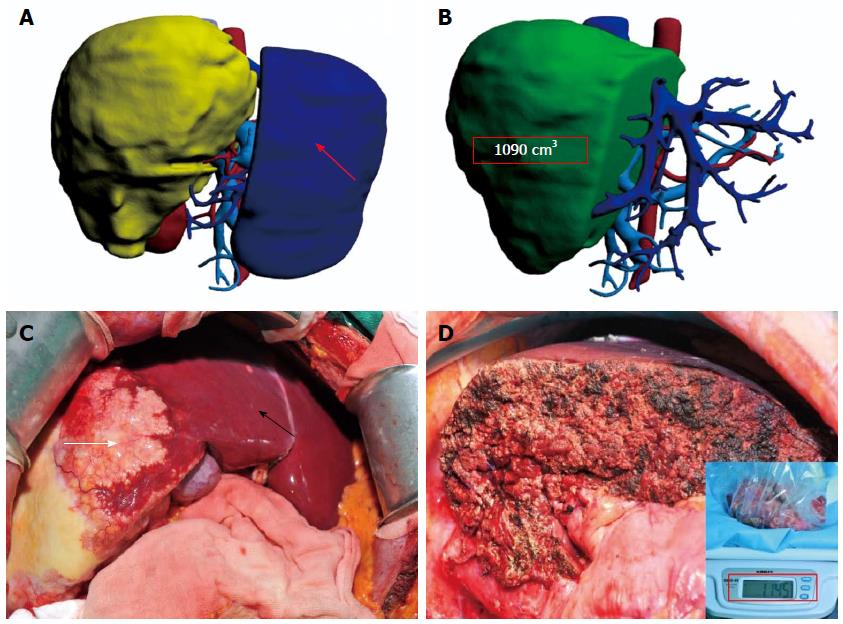
Individualized virtual surgery of the liver was designed based on the reconstructed 3D model for radical resection of the lesion and maximal retention of normal liver tissue. Remnant liver volume was calculated (Figure 2A). The diameter of blood vessels in the section was measured (Figure 2B). A surgical plan was finalized after optimization of resection planes. Estimated liver resection volume in both groups had good correlation with actual graft weight (Group A: r = 0.978; Group B: r = 0.960). All 106 patients in Groups A and B were expected to undergo hepatic resection.
Intraoperative exploration and rapid-freezing results were used to diagnose HAE definitively in all 106 patients, with partial hepatectomy carried out according to the preoperative surgical management plan (Figure 2C). Surgical procedures of all 59 patients in Group A were consistent with their preoperative 3D reconstruction virtual surgery (Figure 2D). Among the 47 cases in Group B, the surgical procedures of four patients were changed due to inconsistency between exploration results and preoperative imaging evaluations. Surgical procedures are shown in Table 3. Patients in both groups were treated using identical liver transection technology. The operation time of Group A was significantly shorter than that of Group B (227.1 ± 51.4 min vs 304.6 ± 88.1 min, P < 0.05). Group A patients demonstrated less intraoperative bleeding (308.1 ± 135.4 vs 458.1 ± 175.4 mL, P < 0.05) and intraoperative blood transfusion (186.4 ± 169.6 vs 289.4 ± 199.2 mL, P < 0.05).
| Surgical procedure | Group A(n = 59) | Group B(n = 47) | P value |
| Major hepatectomy | 31 (52.5) | 28 (59.6) | 0.299 |
| Bisegmentectomy | 6 (10.2) | 7 (14.9) | 0.329 |
| Limited resection | 12 (20.3) | 11 (23.4) | 0.441 |
| Combined with cholecystectomy | 36 (61.0) | 30 (63.8) | 0.463 |
| Combined with choledochojejunostomy | 9 (15.3) | 5 (10.6) | 0.345 |
Routine postoperative fasting and restraint from drinking water were carried out in all patients. Liquid or semi-liquid diets were allowed after recovery of gastrointestinal function. Patients in both groups were given identical intravenous therapy of liver-preserving and nutritional support. Laboratory indices of patients on 3 d after surgery are listed in Table 4; no significant differences were found between the two groups except for significantly higher levels of albumin in Group A (26.3 ± 5.9 g/L vs 22.6 ± 4.3 g/L, P < 0.05). No deaths were reported in the perioperative period. Also, no serious complications (e.g., liver failure, and abdominal bleeding) were observed. Other complications included: biliary fistula in three patients (1 in Group A, 2 in Group B); wound infection in nine (4 in Group A, 5 in Group B); ascites in eight (3 in Group A, 5 in Group B); pleural effusion in nine (4 in Group A, 5 in Group B); pulmonary infection in five (2 in Group A, 3 in Group B); and urinary tract infection in one patient in Group A. The postoperative complications are listed in Table 5. There was no significant difference in the duration of postoperative hospital stay between the two groups (12.8 ± 3.0 d vs 12.0 ± 2.8 d, P > 0.05). All patients were followed for 3-46 (mean, 17.3) mo. There were three deaths: two from cerebrovascular accident, and one from a car accident. Two patients were diagnosed with recurrent lesions by ultrasound and CT in Group B, one of whom was treated with a second surgical procedure and discharged after rehabilitation. One patient refused surgery, and was treated with drugs instead.
| Variable | Group A(n = 59) | Group B(n = 47) | P value |
| AST, U/L, mean ± SD | 141.6 ± 71.9 | 151.9 ± 75.1 | 0.668 |
| ALT, U/L, mean ± SD | 107.2 ± 55.9 | 119.5 ± 70.0 | 0.164 |
| ALB, g/L, mean ± SD | 26.3 ± 5.9 | 22.6 ± 4.3 | 0.033 |
| TBIL, mmol/L, mean ± SD | 74.7 ± 35.3 | 88.2 ± 36.5 | 0.833 |
| DBIL, mmol/L, mean ± SD | 42.3 ± 20.0 | 50.2 ± 25.0 | 0.306 |
| PT, s, mean ± SD | 14.4 ± 2.2 | 14.9 ± 2.4 | 0.267 |
| Variable | Group A (n = 59) | Group B (n = 47) | P value |
| Operating time, min, mean ± SD | 227.1 ± 51.4 | 304.6 ± 88.1 | 0.007 |
| Intraoperative blood loss, mL, mean ± SD | 308.1 ± 135.4 | 458.1 ± 175.4 | 0.028 |
| Intraoperative blood transfusion, mL, mean ± SD | 186.4 ± 169.6 | 289.4 ± 199.2 | 0.040 |
| Postoperative hospital stay | 12.8 ± 3.0 | 12.0 ± 2.8 | 0.519 |
| Perioperative mortality | 0 | 0 | |
| Liver failure | 0 | 0 | |
| Abdominal bleeding | 0 | 0 | |
| Biliary fistula | 1 (1.7) | 2 (4.3) | 0.415 |
| Wound infection | 4 (6.8) | 5 (10.6) | 0.358 |
| Ascites | 3 (5.1) | 5 (10.6) | 0.240 |
| Pleural effusion | 4 (6.8) | 5 (10.6) | 0.358 |
| Pulmonary infection | 2 (3.4) | 3 (6.4) | 0.393 |
| Urinary system infection | 1 (1.7) | 0 (0) | 0.557 |
| Total complications | 15 (25.4) | 19 (40.4) | 0.076 |
Until now, preoperative diagnosis and assessment of HAE have been reliant mainly on ultrasound, CT, and magnetic resonance imaging (MRI). Ultrasound examination usually provides a preliminary diagnosis and approximate evaluation of lesion position. Further diagnosis and evaluation of invasion of conduits inside and outside the liver by lesions often require CT and MRI coupled with post-processing methods of the image (e.g., CTA). These examinations are limited to 2D display, which requires surgeons to reconstruct 3D spatial images based on their clinical experience and knowledge of liver anatomy[9,10]. Eventually, surgical management is based on the severity of lesions.
Computer-aided 3D reconstruction of the liver was applied first for planning of liver resection by Oldhafer et al[11,12] in 1999. Until now, the technology has been used widely in the diagnosis of liver disease and planning of liver surgery[13-15]. For the latter, the strategy involves: surgical assessment of benign/malignant liver tumors; assessment of the safety of living-donor liver transplantation; and evaluation of the anatomical complexity of hepatolithiasis[16-19]. We used 3D reconstruction technology for the preoperative assessment of HAE, and compared it with evaluation using conventional 2D imaging.
Based on the detailed anatomical assessment of 106 cases of HAE, three main disease characteristics were noted. First, most lesions were located in the right lobe of the liver, which can even involve part of the left medial lobe and caudate lobe. Second, the first and second hepatic portal hepatic vein systems often demonstrated different degrees of invasion (coupled with cavernous transformation of the portal vein in some cases). Third, lesions could oppress the retrohepatic inferior vena cava, even wrapping or infringing it when disease was pronounced. Growth characteristics of HAE elicited considerable challenges for radical hepatic resection. Hence, precise preoperative assessment of the lesion and anatomical relationship among different conduits in the liver, as well as selection of the best therapeutic regimen, is crucial.
In our study, the operation time of Group A was obviously shorter than that of Group B, with corresponding reductions in the volume of blood transfusion and intraoperative bleeding volume during surgery. This scenario was probably the result of better knowledge of the lesions and anatomical conditions of hepatic vessels, with the aid of 3D reconstruction. Such knowledge reduced the occurrence of unpredictable intraoperative conditions, thereby making the surgical approach and resection plane clearer. Simultaneously, more precise judgment and recognition of hepatic conduits allowed a shorter operation time, which was one of the reasons for the reduced amount of intraoperative bleeding and blood transfusion. Serum levels of albumin of patients in the two groups were different, but the difference was not significant. The rates of postoperative complications and recurrence of lesions in Group A were lower than those of Group B. These results suggested that 3D reconstruction for preoperative evaluation helped postoperative restoration of liver function, and reduced the incidence of postoperative complications and long-term recurrence.
We therefore believe that the 3D reconstruction technique is superior to the conventional 2D imaging method in the following aspects.
The 3D reconstruction system can provide an individualized model for each patient with comprehensive anatomical information of the liver. 3D reconstruction of an HAE lesion and the liver enables a more thorough understanding of the location and size of the lesion, as well as the adjacent structures. In addition, 3D reconstruction vividly reflects the structural characteristics of the intrahepatic biliary and vascular network, such as the positional relationship among vessels and the diameters, which ensures an accurate preoperative assessment and smooth surgical procedure[20,21].
The 3D reconstruction system is simple and convenient to operate. Surgeons can rapidly obtain liver anatomical information of a patient without having to be proficient in the complicated operational processes in 2D imaging[22,23]. Therefore, the 3D reconstruction system enables higher efficiency in preoperative assessment compared to conventional 2D methods, and is more practical in clinical practice.
The 3D reconstruction system allows surgeons to perform virtual surgery on the 3D model of a liver, which maximizes the human-computer interaction in the course of medical treatment[24,25]. The process is helpful for surgeons to understand fully the specific details about key parts[26,27]. The occurrence of intraoperative incidents, such as biliary and vascular injuries, bleeding, and bile leakage, can be effectively prevented by designing different resection planes and optimizing surgical strategies[28,29].
Accurate anatomical evaluation and precise surgical planning contribute to selection of the best therapeutic methods for HAE[30,31]. We believe that, for preoperative assessment of HAE patients, conventional CTA should be used first, with further 3D assessment conducted if surgery is possible. Further magnetic resonance cholangiopancreatography combined with 3D evaluation should be carried out in case of obvious dilatation of the biliary tract, followed by virtual surgery enabled by 3D technology, to evaluate the feasibility and safety of the procedure. Such 3D reconstruction for preoperative evaluation and surgical planning could increase the chance of success of surgery and reduce operative risk if combined with ultrasound, CT, or MRI. Computer-aided surgical planning systems can be useful for decision making, and are worthy of further exploration.
Hepatic alveolar echinococcosis (HAE) is primarily treated by surgery. A giant HAE lesion can lead to serious damage to the normal structure of intrahepatic vascular and biliary networks, which makes it difficult and risky to perform conventional radical surgery. The lesion can metastasize, in which case the risk of mortality is high, leaving liver transplantation or palliative drug treatment as the only options. So, accurate anatomical evaluation and precise surgical management are crucial for HAE treatment.
Intraoperative navigation systems and three-dimensional (3D)-printed liver replicas based on 3D reconstruction techniques will be excellent tools for liver surgery in the future. The precise 3D models provide practical tools and have a number of possible characteristic applications for surgical planning and medical education.
In this study, 3D reconstruction technology was used for the preoperative assessment of HAE, and compared with evaluation using conventional 2D imaging, and the main characteristics of HAE were observed using the 3D technique.
The IQQA-liver system takes computed tomography (CT) and magnetic resonance images as input, and provides automated 3D segmentation of tumor, liver, liver lobes, as well as vascular and ductal structures. Upon surgeons’ confirmation or local adjustment of such segmentation results, quantitative measurements in terms of the anatomical volume and 3D spatial relationship may be derived to support the physician’s assessment, in addition to 3D visualization. The system further provides “virtual knife” tools for physicians to define target resection/remnant volume, or define vascular branches for local territorial analysis.
Advances in digital techniques has allowed 3D hepatic modeling from CT or MRI images, which provides detailed hepatic anatomy. Virtual surgery, in general, is a virtual reality technique of simulating a surgical procedure, which helps surgeons improve surgical plans and practice surgical processes on 3D models.
The paper is well written and is relevant to the surgical community. Authors compared clinical outcomes of hepatectomy between patients who underwent preoperative virtual hepatectomy and those who did not in order to evaluate the reliability and accuracy of 3D computer reconstruction for liver resection for patients with hepatic alveolar echinococcosis.
P- Reviewer: Cho A, Marescaux J S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Torgerson PR, Keller K, Magnotta M, Ragland N. The global burden of alveolar echinococcosis. PLoS Negl Trop Dis. 2010;4:e722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 309] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 2. | McManus DP, Li Z, Yang S, Gray DJ, Yang YR. Case studies emphasising the difficulties in the diagnosis and management of alveolar echinococcosis in rural China. Parasit Vectors. 2011;4:196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Zhang W, Zhang Z, Wu W, Shi B, Li J, Zhou X, Wen H, McManus DP. Epidemiology and control of echinococcosis in central Asia, with particular reference to the People’s Republic of China. Acta Trop. 2015;141:235-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Giraudoux P, Raoul F, Afonso E, Ziadinov I, Yang Y, Li L, Li T, Quéré JP, Feng X, Wang Q. Transmission ecosystems of Echinococcus multilocularis in China and Central Asia. Parasitology. 2013;140:1655-1666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Hatipoglu S, Bulbuloglu B, Piskin T, Kayaalp C, Yilmaz S. Living donor liver transplantation for alveolar echinococcus is a difficult procedure. Transplant Proc. 2013;45:1028-1030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Kern P, Wen H, Sato N, Vuitton DA, Gruener B, Shao Y, Delabrousse E, Kratzer W, Bresson-Hadni S. WHO classification of alveolar echinococcosis: principles and application. Parasitol Int. 2006;55 Suppl:S283-S287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 205] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 7. | Stavrou GA, Donati M, Ringe KI, Peitgen HO, Oldhafer KJ. Liver remnant hypertrophy induction--how often do we really use it in the time of computer assisted surgery? Adv Med Sci. 2012;57:251-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Saito S, Yamanaka J, Miura K, Nakao N, Nagao T, Sugimoto T, Hirano T, Kuroda N, Iimuro Y, Fujimoto J. A novel 3D hepatectomy simulation based on liver circulation: application to liver resection and transplantation. Hepatology. 2005;41:1297-1304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Fang CH, Huang YP, Chen ML, Lu CM, Li XF, Qiu WF. Digital medical technology based on 64-slice computed tomography in hepatic surgery. Chin Med J (Engl). 2010;123:1149-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 10. | Xie A, Fang C, Huang Y, Fan Y, Pan J, Peng F. Application of three-dimensional reconstruction and visible simulation technique in reoperation of hepatolithiasis. J Gastroenterol Hepatol. 2013;28:248-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Oldhafer KJ, Högemann D, Stamm G, Raab R, Peitgen HO, Galanski M. [3-dimensional (3-D) visualization of the liver for planning extensive liver resections]. Chirurg. 1999;70:233-238. [PubMed] [Cited in This Article: ] |
| 12. | Oldhafer KJ, Preim B, Dörge C, Peitgen HO, Broelsch ChE. [Acceptance of computer-assisted surgery planning in visceral (abdominal) surgery]. Zentralbl Chir. 2002;127:128-133. [PubMed] [Cited in This Article: ] |
| 13. | Ge PL, Du SD, Mao YL. Advances in preoperative assessment of liver function. Hepatobiliary Pancreat Dis Int. 2014;13:361-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Clavien PA, Emond J, Vauthey JN, Belghiti J, Chari RS, Strasberg SM. Protection of the liver during hepatic surgery. J Gastrointest Surg. 2004;8:313-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Radtke A, Nadalin S, Sotiropoulos GC, Molmenti EP, Schroeder T, Valentin-Gamazo C, Lang H, Bockhorn M, Peitgen HO, Broelsch CE. Computer-assisted operative planning in adult living donor liver transplantation: a new way to resolve the dilemma of the middle hepatic vein. World J Surg. 2007;31:175-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Wei L, Zhu ZJ, Lü Y, Jiang WT, Gao W, Zeng ZG, Shen ZY. Application of computer-assisted three-dimensional quantitative assessment and a surgical planning tool for living donor liver transplantation. Chin Med J (Engl). 2013;126:1288-1291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 17. | Fang CH, Liu J, Fan YF, Yang J, Xiang N, Zeng N. Outcomes of hepatectomy for hepatolithiasis based on 3-dimensional reconstruction technique. J Am Coll Surg. 2013;217:280-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Miyamoto R, Oshiro Y, Hashimoto S, Kohno K, Fukunaga K, Oda T, Ohkohchi N. Three-dimensional imaging identified the accessory bile duct in a patient with cholangiocarcinoma. World J Gastroenterol. 2014;20:11451-11455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 18] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Zein NN, Hanouneh IA, Bishop PD, Samaan M, Eghtesad B, Quintini C, Miller C, Yerian L, Klatte R. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19:1304-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 20. | Tang JH, Yan FH, Zhou ML, Xu PJ, Zhou J, Fan J. Evaluation of computer-assisted quantitative volumetric analysis for pre-operative resectability assessment of huge hepatocellular carcinoma. Asian Pac J Cancer Prev. 2013;14:3045-3050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Qian NS, Liao YH, Cai SW, Raut V, Dong JH. Comprehensive application of modern technologies in precise liver resection. Hepatobiliary Pancreat Dis Int. 2013;12:244-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Wang Y, Zhang Y, Peitgen HO, Schenk A, Yuan L, Wei G, Sun Y. Precise local resection for hepatocellular carcinoma based on tumor-surrounding vascular anatomy revealed by 3D analysis. Dig Surg. 2012;29:99-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Urahashi T, Katsuragawa H, Yamamoto M, Ihara Y, Sanada Y, Wakiya T, Mizuta K. Use of 3-dimensional computed hepatic venous visualization for graft outflow venoplasty in adult left living-donor liver transplant. Exp Clin Transplant. 2012;10:350-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Radtke A, Sotiropoulos GC, Molmenti EP, Sgourakis G, Schroeder T, Beckebaum S, Peitgen HO, Cicinnati VR, Broelsch CE, Broering DC. Transhilar passage in right graft live donor liver transplantation: intrahilar anatomy and its impact on operative strategy. Am J Transplant. 2012;12:718-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Peterhans M, Oliveira T, Banz V, Candinas D, Weber S. Computer-assisted liver surgery: clinical applications and technological trends. Crit Rev Biomed Eng. 2012;40:199-220. [PubMed] [Cited in This Article: ] |
| 26. | Takamoto T, Hashimoto T, Ogata S, Inoue K, Maruyama Y, Miyazaki A, Makuuchi M. Planning of anatomical liver segmentectomy and subsegmentectomy with 3-dimensional simulation software. Am J Surg. 2013;206:530-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Mise Y, Tani K, Aoki T, Sakamoto Y, Hasegawa K, Sugawara Y, Kokudo N. Virtual liver resection: computer-assisted operation planning using a three-dimensional liver representation. J Hepatobiliary Pancreat Sci. 2013;20:157-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Simpson AL, Geller DA, Hemming AW, Jarnagin WR, Clements LW, D’Angelica MI, Dumpuri P, Gönen M, Zendejas I, Miga MI. Liver planning software accurately predicts postoperative liver volume and measures early regeneration. J Am Coll Surg. 2014;219:199-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Hayashi H, Beppu T, Okabe H, Kuroki H, Nakagawa S, Imai K, Nitta H, Chikamoto A, Ishiko T, Baba H. Functional assessment versus conventional volumetric assessment in the prediction of operative outcomes after major hepatectomy. Surgery. 2015;157:20-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Dong J, Yang S, Zeng J, Cai S, Ji W, Duan W, Zhang A, Ren W, Xu Y, Tan J. Precision in liver surgery. Semin Liver Dis. 2013;33:189-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Chen G, Li XC, Wu GQ, Wang Y, Fang B, Xiong XF, Yang RG, Tan LW, Zhang SX, Dong JH. The use of virtual reality for the functional simulation of hepatic tumors (case control study). Int J Surg. 2010;8:72-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |