Published online Sep 7, 2015. doi: 10.3748/wjg.v21.i33.9785
Peer-review started: November 30, 2014
First decision: January 8, 2015
Revised: February 6, 2015
Accepted: March 31, 2015
Article in press: March 31, 2015
Published online: September 7, 2015
Processing time: 280 Days and 21.2 Hours
AIM: To evaluate the accuracy of diffusion-weighted imaging (DWI) without bowel preparation, the optimal b value and the changes in apparent diffusion coefficient (ADC) in detecting ulcerative colitis (UC).
METHODS: A total of 20 patients who underwent 3T magnetic resonance imaging (MRI) without bowel preparation and colonoscopy within 24 h were recruited. Biochemical indexes, including C-reactive protein (CRP), erythrocyte sedimentation rate, hemoglobin, leucocytes, platelets, serum iron and albumin, were determined. Biochemical examinations were then performed within 24 h before or after MR colonography was conducted. DWI was performed at various b values (b = 0, 400, 600, 800, and 1000 s/mm2). Two radiologists independently and blindly reviewed conventional- and contrast-enhanced MR images, DWI and ADC maps; these radiologists also determined ADC in each intestinal segment (rectum, sigmoid, left colon, transverse colon, and right colon). Receiver operating characteristic (ROC) analysis was performed to assess the diagnostic performance of DWI hyperintensity from various b factors, ADC values and different radiological signs to detect endoscopic inflammation in the corresponding bowel segment. Optimal ADC threshold was estimated by maximizing the combination of sensitivity and specificity. MR findings were correlated with endoscopic results and clinical markers; these findings were then estimated by ROC analysis.
RESULTS: A total of 100 segments (71 with endoscopic colonic inflammation; 29 normal) were included. The proposed total magnetic resonance score (MR-score-T) was correlated with the total modified Baron score (Baron-T; r = 0.875, P < 0.0001); the segmental MR score (MR-score-S) was correlated with the segmental modified Baron score (Baron-S; r = 0.761, P < 0.0001). MR-score-T was correlated with clinical and biological markers of disease activity (r = 0.445 to 0.831, P < 0.05). MR-score-S > 1 corresponded to endoscopic colonic inflammation with a sensitivity of 85.9%, a specificity of 82.8% and an area under the curve (AUC) of 0.929 (P < 0.0001). The accuracy of DWI hyperintensity was significantly greater at b = 800 than at b = 400, 600, or 1000 s/mm2 (P < 0.05) when endoscopic colonic inflammation was detected. DWI hyperintensity at b = 800 s/mm2 indicated endoscopic colonic inflammation with a sensitivity of 93.0%, a specificity of 79.3% and an AUC of 0.867 (P < 0.0001). Quantitative analysis results revealed that ADC values at b = 800 s/mm2 differed significantly between endoscopic inflamed segment and normal intestinal segment (1.56 ± 0.58 mm2/s vs 2.63 ± 0.46 mm2/s, P < 0.001). The AUC of ADC values was 0.932 (95% confidence interval: 0.881-0.983) when endoscopic inflammation was detected. The threshold ADC value of 2.18 × 10-3 mm2/s indicated that endoscopic inflammation differed from normal intestinal segment with a sensitivity of 89.7% and a specificity of 80.3%.
CONCLUSION: DWI combined with conventional MRI without bowel preparation provides a quantitative strategy to differentiate actively inflamed intestinal segments from the normal mucosa to detect UC.
Core tip: Our results indicated that diffusion-weighted imaging (DWI) provides qualitative and quantitative information when this technique is combined with conventional magnetic resonance imaging without bowel preparation; the combined technique demonstrates a good diagnostic performance to detect colonic inflammation in ulcerative colitis. This technique is completely non-invasive, does not apply ionizing radiation or contrast material injection, does not require any bowel preparation and does not cause discomfort to patients. The optimal b value is 800 s/mm2. DWI hyperintensity at b = 800 s/mm2 detected endoscopic colonic inflammation with a sensitivity of 93.0% and a specificity of 79.3%.
- Citation: Yu LL, Yang HS, Zhang BT, Lv ZW, Wang FR, Zhang CY, Chen WB, Zhang HM. Diffusion-weighted magnetic resonance imaging without bowel preparation for detection of ulcerative colitis. World J Gastroenterol 2015; 21(33): 9785-9792
- URL: https://www.wjgnet.com/1007-9327/full/v21/i33/9785.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i33.9785
Magnetic resonance imaging (MRI) is an excellent technique to accurately detect colorectal cancer[1-3]. MRI has been applied in the diagnosis and follow-up of patients with inflammatory bowel disease[3-15]. For such examinations and certainly for endoscopy, bowel cleansing preparations are required and are often poorly tolerated by patients[16]. Consequently, the use of MRI in clinical practice may be limited.
Only a few studies have reported the use of diffusion-weighted imaging (DWI) in patients with ulcerative colitis (UC)[3,6,10-14,17]. Among these studies, only one[14] reported the value of quantitative DWI to assess inflammatory activity in UC. However, optimal b value of colon DWI to detect colonic inflammation in patients with UC has not yet been published. As such, optimal b value should be determined to produce high-quality apparent diffusion coefficient (ADC) maps that affect the accuracy of ADC measurements and visual imaging interpretations[18].
This study aimed to determine the optimal b value of colon DWI to detect colonic inflammation in patients with UC without bowel preparation at 3T, to evaluate the accuracy of DWI combined with MRI, and to investigate the changes in ADC of patients with UC.
This prospective observational study was conducted with an approval from our institutional review board. Informed consent was also obtained from all of the patients. A total of 23 patients with known or suspected UC underwent magnetic resonance colonography, including DWI without bowel preparation followed by colonoscopy within 24 h, between January 17, 2012 and February 15, 2013. Patients who were diagnosed with UC by colonoscopy were enrolled in the study. These patients did not undergo interval treatment for UC between MRI and colonoscopy. Furthermore, patients were excluded if they were intolerant to colonoscopy or if they suffered from a toxic megacolon, revealed a history of abdominal surgery or experienced other systemic diseases.
The UC clinical score consisted of a modification of the four-category scoring system of the Mayo Clinic[19-22] (Mayo index), namely, rectal bleeding, stool frequency, functional assessment by a patient and global assessment by a physician. Scores ranged from 0 (normal) to 3 (severe disease). Composite scores ranged from 0 (inactive disease) to 12 (severe disease activity). Biochemical indexes, including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), hemoglobin, leucocytes, platelets, serum iron and albumin, were obtained. Biochemical examinations were then performed within 24 h before or after MR colonography was conducted.
MRI examinations were performed using a 3.0 T Philips scanner (Achieva 3.0T, YX, Best, Holland). The following sequences were obtained using an eight-channel, phased-array body coil: (1) axial and coronal balanced turbo field echo with and without fat suppression [repetition time (TR), 3.4 ms; echo time (TE), 1.4 ms; matrix, 224 × 224; flip angle, 45°; slice thickness, 6 mm; gap, 0 mm]; (2) axial and coronal T2-weighted single-shot fast spin echo with and without fat suppression (TR, 2000 ms; TE, 40 ms; matrix, 256 × 256; slice thickness, 6 mm; gap, 0 mm); (3) a 3D fast field echo (FFE) T1 sequence after intravenous administration of 0.2 mL/kg body weight of gadopentetatedimeglumine (Magnevist, Bayer, Germany) at a rate of 2 mL/s for a dynamic study of the axial plane with an arterial phase (25 s after injection) and a portal phase (70 s after injection) and a 2D FFE with fat saturation at 3 min after injection in axial and coronal planes; and (4) axial and/or coronal diffusion-weighted images (b = 0, 400, 600, 800 and 1000 s/mm2; TR, 2357 ms; TE, 62 ms; matrix, 300 × 231; slice thickness, 5 mm; gap, 0 mm; number of signals acquired, the field of view ranged between 32 and 40 cm. Acquisition time for the DWI sequences covering the abdomen and the pelvis ranged from 3 min to 5 min.
DWI was examined at b = 0, 400, 600, 800 and 1000 s/mm2. Two experienced radiologists who were blinded to clinical and endoscopic examination results independently reviewed DWI images and evaluated the radiological signs of DWI hyperintensity. The presence and absence of DWI hyperintensity in a specific segment were rated ‘1’ and ‘0’, respectively. ADC maps were generated from the b factor (0 and 800 s/mm2). To obtain ADC, we magnified the images and placed the oval regions of interest on the largest possible area covering the bowel wall. The measurements were conducted from the area of brightest signal in the bowel wall on the DWI image. The mean of the two ADCs was accepted as ADC of the segment. Based on a comprehensive review of the literature, seven radiological signs were evaluated: (1) DWI hyperintensity (b = 800 s/mm2); (2) rapid gadolinium enhancement after intravenous contrast medium administration (20 s to 25 s after gadolinium infusion); (3) differentiation between the mucosa-submucosa complex and the muscularis; (4) bowel wall thickening (exceeding 5 mm); (5) parietal oedema; (6) the presence of ulceration(s); and (7) comb sign of engorged vasa recta that perpendicularly penetrated the bowel wall (18). These radiological signs were evaluated for each bowel segment as follows: 0 = absence and 1 = presence. The segmental MR-score (MR-score-S) was defined as the sum of the scores of the seven radiological signs for a specific segment. The total MR-score (MR-score-T) was defined as the sum of the MR-score-S for a patient, with values ranging from 0 to 35. MR-scores were independently established by two experienced radiologists who were blinded to the endoscopic data.
Colonoscopy is considered the “gold standard” to detect colonic inflammation in UC. Oral ingestion of 2000 mL to 3000 mL of polyethylene glycol electrolyte solution (Heshuang, China) was used to perform bowel preparation before colonoscopy was conducted. Colonoscopies were performed by two experienced endoscopists who had no prior knowledge of the MRI analysis results. The modified Baron score[19] represents an endoscopic lesion classification. This score ranges from 0 to 4, with 0 for normal mucosa, 1 for granular mucosa with an abnormal vascular pattern, 2 for friable mucosa, 3 for micro-ulceration with spontaneous bleeding, and 4 for gross ulceration. The colon was divided into five sections: rectum, sigmoid, left colon, transverse colon and right colon. A segmental modified Baron score (Baron-S) represents the score of each section. The total modified Baron Score (Baron-T) was defined as the sum of the segmental scores. The result was considered “positive” if Baron-S ≥ 1 and “negative” if Baron-S < 1.
Patients who underwent colonoscopy and were diagnosed with UC were recruited into the analysis. Data were performed with SPSS Statistics version 19.0 and MedCalc version 12.4. All reported P-values were two-sided and P < 0.05 was considered statistically significant.
Receiver operating characteristic (ROC) analysis was performed to assess the diagnostic performance of DWI hyperintensity from various b factors, ADC, MR-score-S and seven radiological signs to detect endoscopic inflammation in the corresponding bowel segment. Analysis was performed to calculate sensitivity, specificity and area under the ROC curve (AUROC) with the associated P-value. The Delong mode was used to compare AUROC. Youden index analysis was performed to estimate the optimal ADC threshold value by maximizing the combination of sensitivity and specificity.
Correlative analysis was performed with Spearman’s correlation coefficients as follows: (1) MR-score-S vs Baron-S; (2) MR-score-T vs Baron-T; (3) MR-score-T vs clinical and biological markers; and (4) Baron-T vs clinical and biological markers. The correlation coefficient of the MR-score was compared with that of the endoscopic scores.
The inter-observer agreement for ADC measurements was performed by two radiologists and calculated with Pearson’s correlation coefficient. Inter-observer agreements between two independent radiologists for the DWI hyperintensity and MR-score were evaluated by kappa statistic.
Among the 23 patients with known or suspected UC, 1 failed to complete a full colonoscopy examination, and 2 were finally diagnosed with Crohn’s disease. Thus, a total of 20 patients were finally recruited in the study.
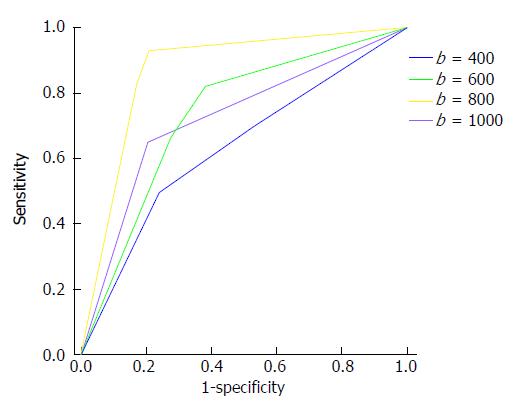
Table 1 presents the sensitivity, specificity and AUROC of DWI hyperintensity at b = 400, 600, 800 and 1000 s/mm2. The DWI hyperintensity at b = 800 s/mm2 detected endoscopic inflammation with a sensitivity of 93.0%, a specificity of 79.3%, and an AUROC of 0.867 (P < 0.0001). The accuracy was significantly greater at b = 800 s/mm2 than at b = 400, 600 or 1000 s/mm2 (P < 0.05; Figure 1). No significant differences in accuracy were found for b = 400, 600 and 1000 s/mm2 (P > 0.05).
| AUROC | Sens. | Spec. | P value | |
| b = 400 s/mm2 | 0.631 | 69.0 | 48.3 | 0.0410 |
| b = 600 s/mm2 | 0.732 | 81.7 | 62.1 | 0.0001 |
| b = 800 s/mm2 | 0.867 | 93.0 | 79.3 | 0.0001 |
| b = 1000 s/mm2 | 0.721 | 64.8 | 79.3 | 0.0010 |
Quantitative analysis results revealed that the mean ADC at b = 800 s/mm2 of the proven endoscopic mucosal inflammation was 1.56 ± 0.58 × 10-3 mm2/s (range, 0.46 × 10-3 mm2/s to 2.50 × 10-3 mm2/s) compared with 2.63 ± 0.46 × 10-3 mm2/s (range, 1.44 × 10-3 mm2/s to 4.03 × 10-3 mm2/s) in normal bowel segments (P < 0.0001). The AUROC was 0.932 (95% confidence interval, 0.881 to 0.983). A threshold ADC value of 2.18 × 10-3 mm2/s could differentiate inflamed bowel from normal bowel segments with a sensitivity of 89.7% and a specificity of 80.3%.
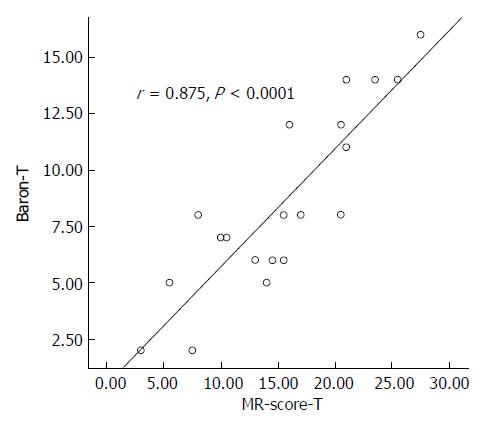
MR-score-T was correlated with Baron-T (r = 0.875, P < 0.0001; Figure 2) and MR-score-S was correlated with Baron-S (r = 0.761, P < 0.0001).
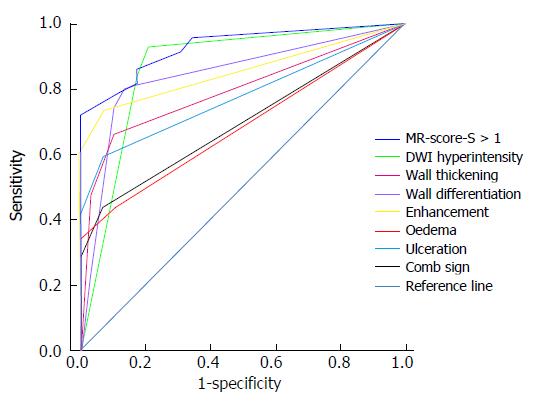
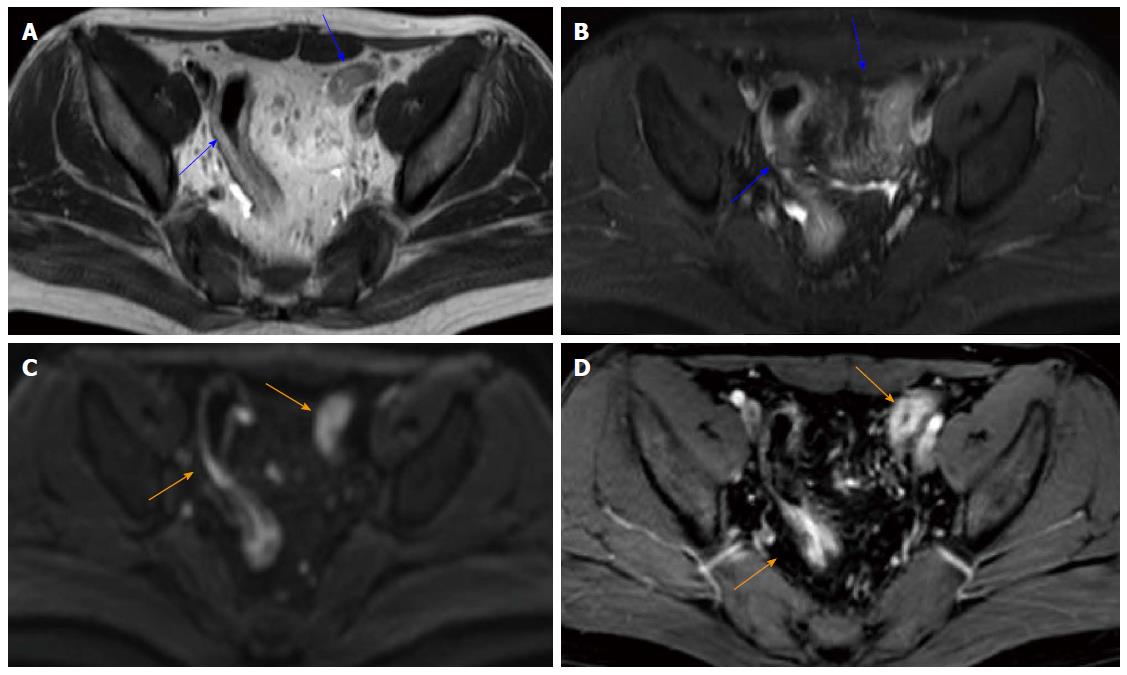
Table 2 and Figure 3 present the sensitivity, specificity, AUROC and ROC of MR-score-S and the seven signs indicating endoscopic inflammation. Figure 4 shows a concrete and representative case. At MR-score-S > 1, endoscopic colonic inflammation could be detected with a sensitivity of 85.9%, a specificity of 82.8% and an AUROC of 0.929 (P < 0.0001). The DWI hyperintensity demonstrated a sensitivity of 93.0% and a specificity of 79.3% to detect endoscopic inflammation with an AUROC of 0.867 (P < 0.0001). With rapid gadolinium enhancement, endoscopic colonic inflammation was detected with a sensitivity of 73.2%, a specificity of 93.1% and an AUROC of 0.853 (P < 0.0001). The accuracy between DWI hyperintensity and rapid gadolinium enhancement (P = 0.78) was not significantly different. Differentiation between the mucosa-sub mucosa complex and the muscles revealed a good sensitivity (80.3%) and specificity (86.2%). The four other signs demonstrated low sensitivities (range: 43.7% to 66.2%) and excellent specificities (range: 89.7% to 93.1%). The presence of oedemas resulted in a decreased accuracy compared with the accuracy of the seven signs indicating endoscopic inflammation. No significant differences in accuracy were observed among other signs.
| ROC analysis | AUROC | Sens. | Spec. | P value |
| MR-score-S > 11 | 0.929 | 85.9 | 82.8 | 0.0001 |
| DWI hyperintensity | 0.867 | 93.0 | 79.3 | 0.0001 |
| Rapid gadolinium enhancement after intravenous contrast medium administration | 0.853 | 73.2 | 93.1 | 0.0001 |
| Bowel wall thickening | 0.793 | 66.2 | 89.7 | 0.0001 |
| Differentiation between the mucosa-submucosa complex and the muscularis | 0.842 | 80.3 | 86.2 | 0.0001 |
| Parietal edema | 0.684 | 43.7 | 89.7 | 0.0040 |
| Ulceration | 0.775 | 59.2 | 93.1 | 0.0001 |
| Comb sign | 0.694 | 43.7 | 93.1 | 0.0020 |
MR-score-T was correlated with Mayo index (r = 0.831, P < 0.0001). Biological indexes included CRP, ESR, hemoglobin, leucocytes, platelets, serum iron and albumin (r = 0.445 to 0.748, P < 0.05). The correlation coefficients between MR-score-T and clinical and biological markers were similar to the corresponding correlation coefficients between Baron-T and the same disease activity markers (Table 3).
| Activity markers | MR-score-T | Baron-T | MR-score-T vs Baron-T | ||
| r | P value1 | r | P value1 | P value2 | |
| Mayo index | 0.831 | 0.0001 | 0.926 | 0.0001 | 0.20 |
| CRP | 0.656 | 0.0020 | 0.886 | 0.0001 | 0.07 |
| ESR | 0.748 | 0.0001 | 0.810 | 0.0001 | 0.64 |
| Hemoglobin | -0.449 | 0.0470 | -0.580 | 0.0070 | 0.60 |
| Leukocytes | 0.481 | 0.0320 | 0.506 | 0.0230 | 0.92 |
| Platelets | 0.445 | 0.0490 | 0.534 | 0.0150 | 0.73 |
| Serum iron | -0.497 | 0.0260 | -0.559 | 0.0100 | 0.80 |
| Albumin | -0.462 | 0.0400 | -0.507 | 0.0220 | 0.86 |
Inter-observer agreements in DWI hyperintensity from various b values were consistent with kappa values ranging from 0.719 to 0.825. The inter-observer agreements were applicable to evaluate MR-score with kappa values ranging from 0.679 to 0.897. The two radiologists’ ADC measurements were compared and Pearson’s correlation coefficient was 0.886 (P < 0.001), thereby indicating an excellent inter-observer agreement.
The selection of the b value should satisfy the following three criteria[23]: (1) clearly display and identify the tissue being examined; (2) effectively inhibit the T2 shine-through effect on DWI; and (3) use b values as high as possible to determine ADC of the tissue being examined for closer to the true diffusion value. A small b value corresponded to high signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) of DWI images. However, the influence was more distinct on ADC with the T2 shine-through effect, perfusion effects and presence of macroscopic motion. Conversely, a large b value indicated that ADC was closer to the real diffusion values of the tissue. However, susceptibility artefacts and geometric deformation of images likely decreased significantly the SNR and the CNR of images. Therefore, the selection of the b value should weigh the two aspects of the real diffusion values of tissue and image quality. Oto et al[9] evaluated the value of DWI (b = 600 s/mm2) and investigated changes in ADC values in inflamed bowels in patients with Crohn’s disease at 1.5 T. Oussalah et al[6] also found that the b factor is fixed at 600 s/mm2 with a 1.5 T scanner in UC and Crohn’s disease. Kılıçkesmez et al[14] evaluated 28 patients with UC by DW-MRI with b = 0, 500 and 1000 s/mm2 on a 1.5 T scanner. The current study defined the range of the b value from 0 and 400 s/mm2 to 1000 s/mm2 by referring to previous studies. In the current study, DWI hyperintensity at b = 800 s/mm2 demonstrated the most efficient diagnostic performance to detect colonic inflammation in UC. The difference in the b value between the results of the current study and that described in a previous study may be related to differences in field strength and uniformity of the main magnetic field.
Oto et al[9] found statistically significant differences between the ADC values of inflamed and normal bowel segments of patients with Crohn’s disease (0.47 × 10-3 mm²/s to 2.60 × 10-3 mm²/s and 1.39 × 10-3 mm²/s to 4.03 × 10-3 mm²/s for inflamed and normal segments, respectively; P < 0.05). Kiryu et al[7] also found that the ADC values of the small and large bowel of patients with active disease were lower than those in patients with inactive disease (1.61 ± 0.44 × 10-3 mm2/s vs 2.56 ± 0.51 × 10-3 mm²/s for the small bowel and 1.52 ± 0.43 × 10-3± 10-3 mm²/s vs 2.31 ± 0.59 × 10-3 mm²/s for the large bowel; P < 0.001). Kılıçkesmez et al[14] found that the ADC values of the rectum are different (P = 0.009) between patients in active (1.08 ± 0.14 × 10-³ mm2/s) and sub-acute phases (1.13 ± 0.23 × 10-3 mm2/s) of the disease and those in remission (1.29 ± 0.17 × 10-³ mm2/s). In the current study, the mean ADC value of proven endoscopic inflamed bowels was 1.56 ± 0.58 × 10-3 mm2/s compared with 2.63 ± 0.46 × 10-3 mm2/s in normal bowel segments (P < 0.0001). In these studies, radiologists should be aware of possible overlaps of ADC values that may lead to misdiagnoses when only DWI is interpreted[24]. The usefulness of ADC for long-term follow-up of patients with UC warrants further investigation.
DWI is a method in which the signal required to produce MR image is determined by the “mobility of water”[25]. Diffusivity measurements are characterized by multiple components related to tissue cellularity and organisation, integrity of cell membranes, extracellular space tortuosity and perfusion[26]. Endoscopic biopsy is considered the gold standard to detect and quantify UC; invasiveness, patient discomfort, perforation risk and poor patient acceptance of colonoscopy have prompted researchers to investigate alternatives for diagnosing and characterizing UC. In MR or endoscopy examination, oral and rectal bowel cleansing preparations are often poorly tolerated by patients[27,28]. The technique used in the current study did not require oral or rectal preparation and fasting; the duration of the procedure was relatively short (approximately 20 min for the whole examination, including patient setup, routine MR and DWI imaging). In the current study, DWI hyperintensity exhibited the same accuracy as rapid gadolinium enhancement to detect endoscopic inflammation in UC; this result suggested that the DWI sequence could replace gadolinium injection in detecting inflammatory colonic segments in UC. In other studies, DWI hyperintensity also showed a high accuracy[6-8]. DWI combined with MRI without bowel preparation represents a feasible tool. This technique is completely non-invasive, does not apply ionizing radiation[29,30] or contrast material injection, does not require any bowel preparation, and does not cause discomfort to patients. Bowel preparation has also been associated with acute exacerbation of UC. Diagnostic methods that do not require bowel preparation could avoid this potential complication. Therefore, the proposed technique can be easily combined with conventional MR examination protocol because of short duration.
Our study showed several limitations, such as small patient population. With our most efficient efforts to magnify images and use oval regions of interest to exclusively cover the bowel wall, the possibility of a partial volume effect was minimised. However, it could not be completely excluded, especially from ADC measurements of the normal bowel wall.
In conclusion, DWI combined with conventional MRI without bowel preparation yielded qualitative and quantitative information; our result demonstrated a good diagnostic performance in detecting colonic inflammation in UC.
Magnetic resonance imaging (MRI) is an excellent technique to accurately detect colorectal cancer. MRI has been applied to diagnose and follow patients with inflammatory bowel disease. In such examinations and endoscopy, bowel cleansing preparations are required and often poorly tolerated by patients. This procedure may limit the use of MRI in clinical practice.
Only a few studies have reported the use of diffusion-weighted imaging (DWI) in patients with ulcerative colitis (UC). Among these studies, only one reported the value of quantitative diffusion-weighted MRI in the assessment of the inflammatory activity in UC. The optimal b value of colon DWI to detect colonic inflammation in patients with UC has not been published.
This results indicated that DWI combined with conventional MRI without bowel preparation yielded qualitative and quantitative information; this study demonstrated good diagnostic performance to detect colonic inflammation in UC.
This technique is completely non-invasive, does not apply ionizing radiation or contrast material injection, does not require any bowel preparation, and does not cause discomfort to patients. Diagnostic methods that do not require bowel preparation could avoid acute exacerbation. This procedure can be easily added to conventional MR examination protocol because of short duration.
The segmental MR-score (MR-score-S) is defined as the sum of the scores of different radiological signs for a specific segment. The total MR-score was defined as the sum of MR-score-S for a patient.
DWI combined with conventional MRI without bowel preparation provided a quantitative technique to differentiate actively inflamed intestinal segments from the normal mucosa to detect UC.
P- Reviewer: Hayes MJ, Kim ES S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Solak A, Genç B, Solak I, Kalaycıoğlu S, Sahin N, Yalaz S, Sivrikoz ON. The value of diffusion-weighted magnetic resonance imaging in the differential diagnosis in diffuse bowel wall thickening. Turk J Gastroenterol. 2013;24:154-160. [PubMed] |
| 2. | Ichikawa T, Erturk SM, Motosugi U, Sou H, Iino H, Araki T, Fujii H. High-B-value diffusion-weighted MRI in colorectal cancer. AJR Am J Roentgenol. 2006;187:181-184. [PubMed] |
| 3. | Kilickesmez O, Atilla S, Soylu A, Tasdelen N, Bayramoglu S, Cimilli T, Gurmen N. Diffusion-weighted imaging of the rectosigmoid colon: preliminary findings. J Comput Assist Tomogr. 2009;33:863-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Koh DM, Miao Y, Chinn RJ, Amin Z, Zeegen R, Westaby D, Healy JC. MR imaging evaluation of the activity of Crohn’s disease. AJR Am J Roentgenol. 2001;177:1325-1332. [PubMed] |
| 5. | Maccioni F, Patak MA, Signore A, Laghi A. New frontiers of MRI in Crohn’s disease: motility imaging, diffusion-weighted imaging, perfusion MRI, MR spectroscopy, molecular imaging, and hybrid imaging (PET/MRI). Abdom Imaging. 2012;37:974-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Oussalah A, Laurent V, Bruot O, Bressenot A, Bigard MA, Régent D, Peyrin-Biroulet L. Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut. 2010;59:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Kiryu S, Dodanuki K, Takao H, Watanabe M, Inoue Y, Takazoe M, Sahara R, Unuma K, Ohtomo K. Free-breathing diffusion-weighted imaging for the assessment of inflammatory activity in Crohn’s disease. J Magn Reson Imaging. 2009;29:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Oto A, Kayhan A, Williams JT, Fan X, Yun L, Arkani S, Rubin DT. Active Crohn’s disease in the small bowel: evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging. 2011;33:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Oto A, Zhu F, Kulkarni K, Karczmar GS, Turner JR, Rubin D. Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn’s disease. Acad Radiol. 2009;16:597-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Langhorst J, Kühle CA, Ajaj W, Nüfer M, Barkhausen J, Michalsen A, Dobos GJ, Lauenstein TC. MR colonography without bowel purgation for the assessment of inflammatory bowel diseases: diagnostic accuracy and patient acceptance. Inflamm Bowel Dis. 2007;13:1001-1008. [PubMed] |
| 11. | Gandolfi L. Comparison of magnetic resonance imaging and endoscopy in distinguishing the type and severity of inflammatory bowel disease. Gastrointest Endosc. 1996;43:86-87. [PubMed] |
| 12. | Madsen SM, Thomsen HS, Munkholm P, Dorph S, Schlichting P. Active Crohn’s disease and ulcerative colitis evaluated by low-field magnetic resonance imaging. Scand J Gastroenterol. 1998;33:1193-1200. [PubMed] |
| 13. | Rimola J, Rodríguez S, García-Bosch O, Ricart E, Pagès M, Pellisé M, Ayuso C, Panés J. Role of 3.0-T MR colonography in the evaluation of inflammatory bowel disease. Radiographics. 2009;29:701-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Kılıçkesmez O, Soylu A, Yaşar N, Demirbaş T, Dolapçıoğlu C, Poturoğlu S, Sevindir I, Cimilli T. Is quantitative diffusion-weighted MRI a reliable method in the assessment of the inflammatory activity in ulcerative colitis? Diagn Interv Radiol. 2010;16:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 15. | Mentzel HJ, Reinsch S, Kurzai M, Stenzel M. Magnetic resonance imaging in children and adolescents with chronic inflammatory bowel disease. World J Gastroenterol. 2014;20:1180-1191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Achiam MP, Løgager V, Chabanova E, Thomsen HS, Rosenberg J. Patient acceptance of MR colonography with improved fecal tagging versus conventional colonoscopy. Eur J Radiol. 2010;73:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Aoyagi T, Shuto K, Okazumi S, Miyauchi H, Kazama T, Matsubara H. Evaluation of ulcerative colitis using diffusion-weighted imaging. Hepatogastroenterology. 2010;57:468-471. [PubMed] |
| 18. | Saritas EU, Lee JH, Nishimura DG. SNR dependence of optimal parameters for apparent diffusion coefficient measurements. IEEE Trans Med Imaging. 2011;30:424-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, Dubé R, Cohen A, Steinhart AH, Landau S. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499-2507. [PubMed] |
| 20. | Bewtra M. An optimized patient-reported ulcerative colitis disease activity measure derived from the Mayo Score and the Simple Clinical Colitis Activity Index. Inflamm Bowel Dis. 2015;21:E1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Walmsley RS. Comment on an optimized patient-reported ulcerative colitis disease activity measure derived from the mayo score and the simple clinical colitis activity index. Inflamm Bowel Dis. 2014;20:E25-E26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Bewtra M, Brensinger CM, Tomov VT, Hoang TB, Sokach CE, Siegel CA, Lewis JD. An optimized patient-reported ulcerative colitis disease activity measure derived from the Mayo score and the simple clinical colitis activity index. Inflamm Bowel Dis. 2014;20:1070-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Li W, Li D, Liu H, Zhou R, Feng C, Ma Y, Yu T. [3.0T MR diffusion-weighted imaging: evaluating diagnosis potency of pulmonary solid benign lesions and malignant tumors and optimizing b value]. Zhongguo Fei Ai Za Zhi. 2011;14:853-857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Erden A. Are we expecting too much from the diffusion-weighted MRI? Turk J Gastroenterol. 2013;24:85-87. [PubMed] |
| 25. | Laurent V, Trausch G, Bruot O, Olivier P, Felblinger J, Régent D. Comparative study of two whole-body imaging techniques in the case of melanoma metastases: advantages of multi-contrast MRI examination including a diffusion-weighted sequence in comparison with PET-CT. Eur J Radiol. 2010;75:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics. 2009;29:1797-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 27. | Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther. 2007;25:373-384. [PubMed] |
| 28. | Florie J, Birnie E, van Gelder RE, Jensch S, Haberkorn B, Bartelsman JF, van der Sluys Veer A, Snel P, van der Hulst VP, Bonsel GJ. MR colonography with limited bowel preparation: patient acceptance compared with that of full-preparation colonoscopy. Radiology. 2007;245:150-159. [PubMed] |
| 29. | Cakmakci E, Erturk SM, Cakmakci S, Bayram A, Tokgoz S, Caliskan KC, Celebi I. Comparison of the results of computerized tomographic and diffusion-weighted magnetic resonance imaging techniques in inflammatory bowel diseases. Quant Imaging Med Surg. 2013;3:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Peloquin JM, Pardi DS, Sandborn WJ, Fletcher JG, McCollough CH, Schueler BA, Kofler JA, Enders FT, Achenbach SJ, Loftus EV. Diagnostic ionizing radiation exposure in a population-based cohort of patients with inflammatory bowel disease. Am J Gastroenterol. 2008;103:2015-2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |