Published online Aug 21, 2015. doi: 10.3748/wjg.v21.i31.9442
Peer-review started: April 9, 2015
First decision: April 23, 2015
Revised: May 11, 2015
Accepted: June 26, 2015
Article in press: June 26, 2015
Published online: August 21, 2015
A pancreatic paraganglioma is a rare neoplasm that is difficult to distinguish from a pancreatic neuroendocrine tumour. Here we present a case of pancreatic paraganglioma that was surgically resected following preoperative diagnosis of a pancreatic neuroendocrine tumour. Careful evaluation of the endoscopic ultrasonography findings revealed abundant draining vessels, which could have led to a correct preoperative diagnosis of pancreatic paraganglioma.
Core tip: Pancreatic paraganglioma is a rare disease, which has been reported only in 24 cases ever. Invasive procedures toward paragangliomas carry the potential risk of catastrophic complications due to unexpected release of large quantities of catecholamines. An accurate diagnosis of paraganglioma, therefore, is important before invasive procedures. Draining vessels from the tumour are sometimes observed in a general paraganglioma. However, it is still unclear in the pancreatic paraganglioma. In the present report, usefulness of draining vessels for a diagnosis of pancreatic paraganglioma was investigated by reviewing past cases.
- Citation: Misumi Y, Fujisawa T, Hashimoto H, Kagawa K, Noie T, Chiba H, Horiuchi H, Harihara Y, Matsuhashi N. Pancreatic paraganglioma with draining vessels. World J Gastroenterol 2015; 21(31): 9442-9447
- URL: https://www.wjgnet.com/1007-9327/full/v21/i31/9442.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i31.9442
Paragangliomas are rare catecholamine-secreting neuroendocrine tumours that arise from neuroendocrine cells of the extra-adrenal autonomic paraganglia and affect about 1 in 2000000 of the population[1]. The head, neck, and retroperitoneum are the most commonly affected sites, but pancreatic paragangliomas are extremely rare[2]. To our knowledge, only 24 cases of pancreatic paraganglioma have been previously reported in the English literature (see Table 1)[1-20]. In these previous reports, the pancreatic paragangliomas were often preoperatively misdiagnosed as pancreatic neuroendocrine tumours (pNETs) because the radiological characteristics of pancreatic paragangliomas resemble those of pNETs. We herein report a patient with a pancreatic paraganglioma, which was surgically resected following a preoperative diagnosis of a pNET, and discuss features associated with the disorder.
| Age(yr) | Sex | Tumour size (cm) | Location in pancreas | Preoperative diagnosis | Presence/absence of draining vessels | Outcome | Ref. |
| 62 | M | 1.5 | Body | - | NE | Autopsy | [3] |
| 75 | F | 15 | Tail | Pancreatic cyst | NE | - | [4] |
| 70 | F | 3 | Head | Pancreatic cyst | NE | - | [4] |
| 72 | F | 14 | Head | Cystadenoma | NE | 2 yr alive | [5] |
| 47 | M | 10 | Body | Pancreatic cyst | NE | 6 yr alive | [6] |
| - | - | - | Head | - | NE | 2 yr alive | [7] |
| - | - | - | Head | - | NE | 4 yr alive | [7] |
| 45 | F | 8 | Head | Retroperitoneal tumour | NE | 5 yr alive | [8] |
| 58 | M | 8 | Head | Neuroendocrine tumour | NE | - | [8] |
| 61 | M | 2.5 | Uncus | Neuroendocrine tumour | Absent in the presented figures | 5 yr alive | [9] |
| 85 | M | 6 | Head | Neuroendocrine tumour | NE | - | [10] |
| 72 | F | 4 | Uncus | Nonfunctional neuroendocrine tumour | Absent in the presented figures | - | [11] |
| 57 | F | 6.5 | Head | Non-functioning islet cell tumour | Stated as present | - | [12] |
| 57 | F | 2 | Uncus | Neuroendocrine tumour | NE | 4 yr alive | [1] |
| 50 | M | 3 | Head | Extra-adrenal paraganglioma | Present in the presented figures | - | [13] |
| 51 | F | 5 | Uncus | Pancreatic cancer | NE | 3 yr alive | [14] |
| 40 | F | 4.5 | Uncus | - | Stated as absent | - | [15] |
| 66 | M | 6 | Head | - | Absent in the presented figures | 14 mo alive | [2] |
| 65 | F | 2 | Uncus | - | NE | - | [16] |
| 30 | F | 6.4 | Tail | - | NE | Died 34 h after surgery | [17] |
| 19 | F | 9 | Head | Sarcoma | Present in the presented figures | - | [18] |
| 55 | F | 19 | Tail | Malignant pancreatic tumour | NE | - | [19] |
| 50 | F | 6 | Head | Paraganglioma, fine needle aspiration | NE | 4 yr alive | [20] |
| 63 | M | 4 | Head | Functional pancreatic paraganglioma | NE | - | [20] |
| 47 | F | 1.5 | Head | Nonfunctional neuroendocrine tumour | Present | 1 yr alive | Present case |
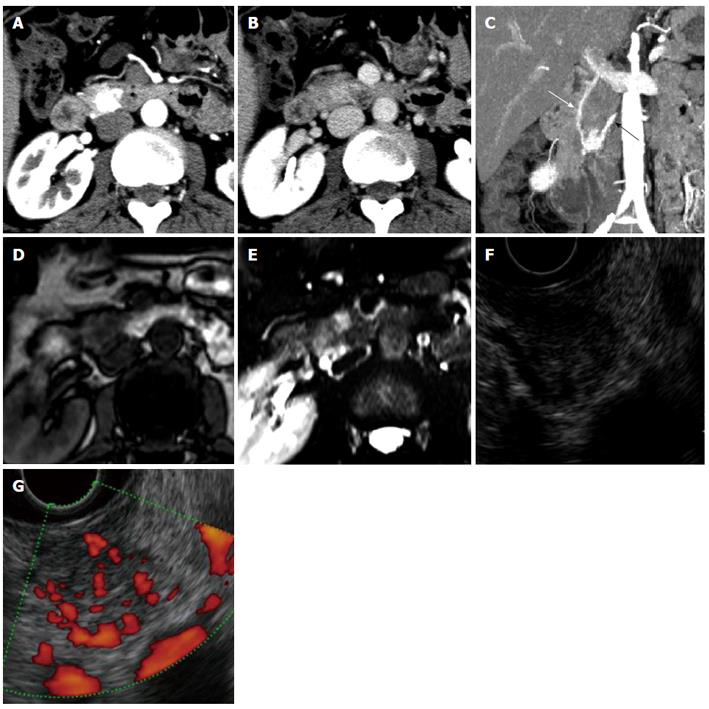
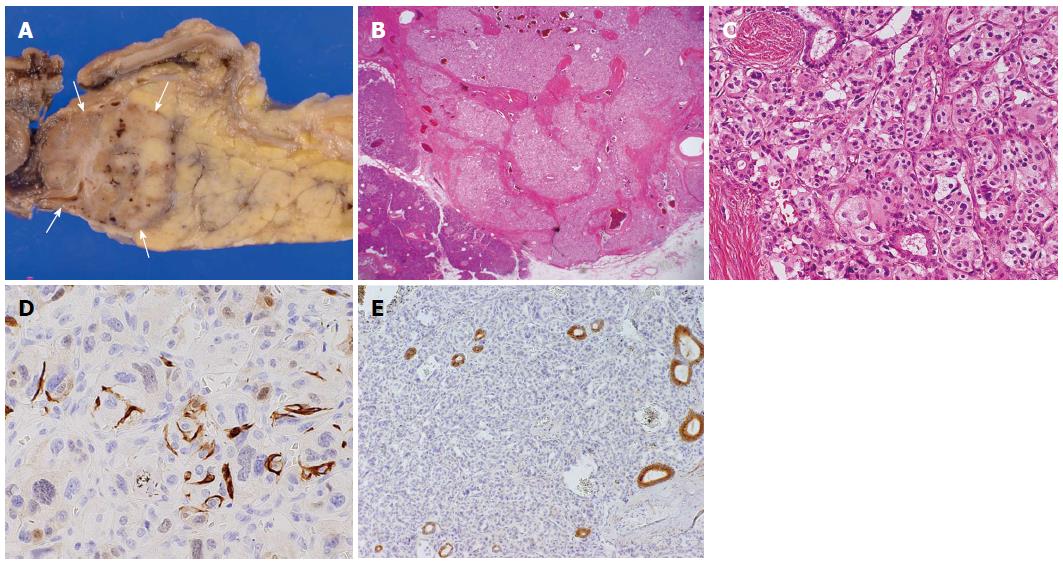
An asymptomatic 47-year-old woman with a pancreatic tumour that was detected by ultrasonography was referred to our hospital. The patient’s past medical history and physical examination were unremarkable. Initial laboratory studies, including levels of tumour markers such as carcinoembryonic antigen, carbohydrate antigen 19-9, and α-fetoprotein, showed no abnormalities. An examination of pancreatic endocrine hormones showed that the insulin level was slightly elevated [14.6 μU/mL (5-10 μU/mL)], but the elevation was considered non-specific because blood glucose (108 mg/dL) and C-peptide levels [3.30 (0.78-5.19) ng/dL] were within normal levels. Levels of other hormones were also within their normal ranges. Transabdominal ultrasonography showed a 1.5-cm low-echoic tumour at the pancreas head. Dynamic computed tomography (CT) also revealed a 1.5-cm well demarcated tumour at the pancreas head (Figure 1A and B). The tumour was very strongly enhanced in the arterial phase and still faintly enhanced in the portal vein phase. Maximum intensity projection of the arterial phase in a coronal view clearly revealed the tumour feeding artery from the inferior pancreaticoduodenal artery and the draining vein into the portal vein (Figure 1C). No abnormalities were found in the bile duct, pancreatic duct, or liver. Compared with contiguous pancreatic parenchyma, the tumour appeared as a low-intensity lesion in a T1-weighted magnetic resonance image (MRI) and as a high-intensity lesion in a T2-weighted MRI (Figure 1D and E, respectively). Endoscopic ultrasonography (EUS) showed a well demarcated, low, and uneven echoic tumour surrounded by draining vessels emanating from the tumour (Figure 1F and G). We did not attempt EUS-guided fine needle aspiration (FNA) for a pathological examination because of the possible risk of bleeding due to the extremely abundant vascularity of the tumour. On the basis of these imaging findings, we made a preoperative diagnosis of a nonfunctional pNET. Pancreaticoduodenectomy was performed. The resected specimen included a tumour measuring 1.5 cm × 1.2 cm (Figure 2A). The tumour was located at the caudal part of pancreas head and partially adjacent to the second portion of duodenum. The tumour was isolated from major arteries and veins. Histological examination revealed a classical Zellballen pattern, with nests of cells surrounded by thick capsules (Figures 2B and C). The tumour was surrounded by normal pancreatic parenchyma. Immunohistochemistry examination of the tumour cells showed positive staining for CD56, synaptophysin, and chromogranin A and negative staining for insulin, glucagon, gastrin, and somatostatin. The tumour cells were surrounded by S-100 protein-positive sustentacular cells (Figure 2D). These results suggested that the tumour cells were differentiated toward neuroendocrine cells, but did not express any islet hormones. Epithelial membrane antigen staining was negative in the tumour cells but positive in the pancreatic ducts remaining within the tumour (Figure 2E). This result indicated that branches of the pancreatic duct were within the tumour. Taking into consideration the tumour location and pancreatic ducts remaining within the tumour, we inferred that the tumour arose from the pancreas. On the basis of these pathological findings, a diagnosis of pancreatic paraganglioma was established. The patient was followed for more than one year after surgery, but no recurrence was confirmed.
Pancreatic paragangliomas are radiologically similar to pNETs. In fact, the case reported here and 6 of the 24 other cases reported in the literature were preoperatively misdiagnosed as pNETs[1,8-12]. Fortunately, no complications occurred during the preoperative examinations or surgical procedures in the present case. However, invasive examinations and surgery of paragangliomas carry the potential risk of catastrophic complications due to unexpected release of large quantities of catecholamines. For example, a patient with pancreatic paraganglioma, who was misdiagnosed preoperatively as having pancreatic cancer, died 34 h after surgery because of unanticipated catecholamine release[17]. The use of EUS-guided FNA without preparations against catecholamine release could also be dangerous. These dangers highlight the importance of an accurate diagnosis of pancreatic paraganglioma.
Preoperative imaging may provide guidance for an accurate diagnosis. Areas of signal flow void (referred to as a “salt and pepper” pattern[21]) are often observed on MRIs of highly vascularized paragangliomas. Additionally, enlarged feeding arteries and early contrast filling of the draining veins are observed on dynamic CT images. This feature of draining veins has been reported to be useful in distinguishing pancreatic paraganglioma from pNET[12]. In the present case, the draining vein was confirmed by dynamic CT, but “salt and pepper” pattern was absent on the MRI. In the 24 other cases (Table 1), one has been reported to have draining veins[12], and one has been reported to be devoid of such veins[15]. The presence or absence of draining veins was not mentioned in the text of the reports of the remaining 22 cases, but examination of images presented in the reports suggest that two cases[13,18] had and three cases[2,9,11] did not have draining veins (presented material did not allow evaluation of draining veins in the remaining 17 cases). Formation of the tumour draining vasculature is supposed to depend on a variety of growth factors secreted from the tumour itself[22]. Most pancreatic paragangliomas seem to have a characteristic to induce abundant draining veins, although details of the mechanism of the vessel induction remain to be elucidated. In summary, draining veins could be observed in 50% (four of eight) of evaluable cases. We conclude that evaluation of imaging findings, especially identifying draining veins around the tumour, may be useful in making a correct preoperative diagnosis of pancreatic paraganglioma.
A 47-year-old woman with a pancreatic tumour detected by ultrasonography was referred to our hospital.
The patient showed no symptom.
Solid tumors (adenocarcinoma, neuroendocrine tumours, Solid-pseudopapillary tumor, acinar cell carcinoma, and paraganglioma), cystic tumors (serous cystic neoplasm).
Laboratory studies, including levels of tumour markers such as carcinoembryonic antigen, carbohydrate antigen 19-9, and α-fetoprotein, showed no abnormalities, but insulin level was slightly elevated [14.6 μU/mL].
Computed tomography showed a 1.5-cm well demarcated tumour at the pancreas head, which was very strongly enhanced in the arterial phase and still faintly enhanced in the portal vein phase.
Histological examination showed a classical Zellballen pattern, and tumour cells of positive staining for CD56, synaptophysin, and chromogranin A, that were surrounded by S-100 protein-positive sustentacular cells.
The patient received pancreaticoduodenectomy.
Pancreatic paraganglioma has been reported only in 24 cases ever.
Paraganglioma is rare catecholamine-secreting neuroendocrine tumour that arises from neuroendocrine cells of the extra-adrenal autonomic paraganglia
Identifying draining veins around the tumour is useful in making a correct preoperative diagnosis of pancreatic paraganglioma.
The authors have summarized 25 cases of pancreatic paraganglioma including the present case and reported usefulness of tumour draining vessels for distinguishing pancreatic paraganglioma from pancreatic neuroendocrine tumour.
P- Reviewer: Koike Y, Sinha R, Takahashi T, Verbeke CS S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Tsukada A, Ishizaki Y, Nobukawa B, Kawasaki S. Paraganglioma of the pancreas: a case report and review of the literature. Pancreas. 2008;36:214-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Lightfoot N, Santos P, Nikfarjam M. Paraganglioma mimicking a pancreatic neoplasm. JOP. 2011;12:259-261. [PubMed] [Cited in This Article: ] |
| 3. | Goodof I, Lischer C. Tumor of the carotid body and the pancreas. Arch Pathol. 1943;35:6. [Cited in This Article: ] |
| 4. | Bartley O, Ekdahl PH, Hultén L. Paraganglioma simulating pancreatic cyst. Acta Chir Scand. 1966;132:289-297. [PubMed] [Cited in This Article: ] |
| 5. | Cope C, Greenberg SH, Vidal JJ, Cohen EA. Nonfunctioning nonchromaffin paraganglioma of the pancreas. Arch Surg. 1974;109:440-442. [PubMed] [Cited in This Article: ] |
| 6. | Zamir O, Amir G, Lernau O, Ne’eman Z, Nissan S. Nonfunctional paraganglioma of the pancreas. Am J Gastroenterol. 1984;79:761-763. [PubMed] [Cited in This Article: ] |
| 7. | Howard JM, Jordan JL, Reber HA. Surgical disease of the pancreas. Philadelphia: Lea and Febiger 1987; . [Cited in This Article: ] |
| 8. | Malthouse SR, Robinson L, Rankin SC. Ultrasonic and computed tomographic appearances of paraganglioma simulating pancreatic mass. Clin Radiol. 1992;45:271-272. [PubMed] [Cited in This Article: ] |
| 9. | Fujino Y, Nagata Y, Ogino K, Watahiki H, Ogawa H, Saitoh Y. Nonfunctional paraganglioma of the pancreas: report of a case. Surg Today. 1998;28:209-212. [PubMed] [Cited in This Article: ] |
| 10. | Parithivel VS, Niazi M, Malhotra AK, Swaminathan K, Kaul A, Shah AK. Paraganglioma of the pancreas: literature review and case report. Dig Dis Sci. 2000;45:438-441. [PubMed] [Cited in This Article: ] |
| 11. | Ohkawara T, Naruse H, Takeda H, Asaka M. Primary paraganglioma of the head of pancreas: contribution of combinatorial image analyses to the diagnosis of disease. Intern Med. 2005;44:1195-1196. [PubMed] [Cited in This Article: ] |
| 12. | Kim SY, Byun JH, Choi G, Yu E, Choi EK, Park SH, Lee MG. A case of primary paraganglioma that arose in the pancreas: the Color Doppler ultrasonography and dynamic CT features. Korean J Radiol. 2008;9 Suppl:S18-S21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Sangster G, Do D, Previgliano C, Li B, LaFrance D, Heldmann M. Primary retroperitoneal paraganglioma simulating a pancreatic mass: a case report and review of the literature. HPB Surg. 2010;2010:645728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Zengel B, Alacacioglu A, Yagci A, Postaci H, Erdinc I, Ozguzer A, Denecli A. Primary paraganglioma of the pancreas: Review of literature and a case report. Firat Tip Dergisi. 2010;15:3. [Cited in This Article: ] |
| 15. | He J, Zhao F, Li H, Zhou K, Zhu B. Pancreatic paraganglioma: A case report of CT manifestations and literature review. Quant Imaging Med Surg. 2011;1:41-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 12] [Reference Citation Analysis (0)] |
| 16. | Higa B, Kapur U. Malignant paraganglioma of the pancreas. Pathology. 2012;44:53-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Wang ZL, Fu L, Zhang Y, Babu SR, Tian B. An asymptomatic pheochromocytoma originating from the tail of the pancreas. Pancreas. 2012;41:165-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Al-Jiffry BO, Alnemary Y, Khayat SH, Haiba M, Hatem M. Malignant extra-adrenal pancreatic paraganglioma: case report and literature review. BMC Cancer. 2013;13:486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Borgohain M, Gogoi G, Das D, Biswas M. Pancreatic paraganglioma: An extremely rare entity and crucial role of immunohistochemistry for diagnosis. Indian J Endocrinol Metab. 2013;17:917-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Zhang L, Liao Q, Hu Y, Zhao Y. Paraganglioma of the pancreas: a potentially functional and malignant tumor. World J Surg Oncol. 2014;12:218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Wieneke JA, Smith A. Paraganglioma: carotid body tumor. Head Neck Pathol. 2009;3:303-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Ruddell A, Croft A, Kelly-Spratt K, Furuya M, Kemp CJ. Tumors induce coordinate growth of artery, vein, and lymphatic vessel triads. BMC Cancer. 2014;14:354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |