Copyright
©The Author(s) 2015.
World J Gastroenterol. Aug 14, 2015; 21(30): 9163-9174
Published online Aug 14, 2015. doi: 10.3748/wjg.v21.i30.9163
Published online Aug 14, 2015. doi: 10.3748/wjg.v21.i30.9163
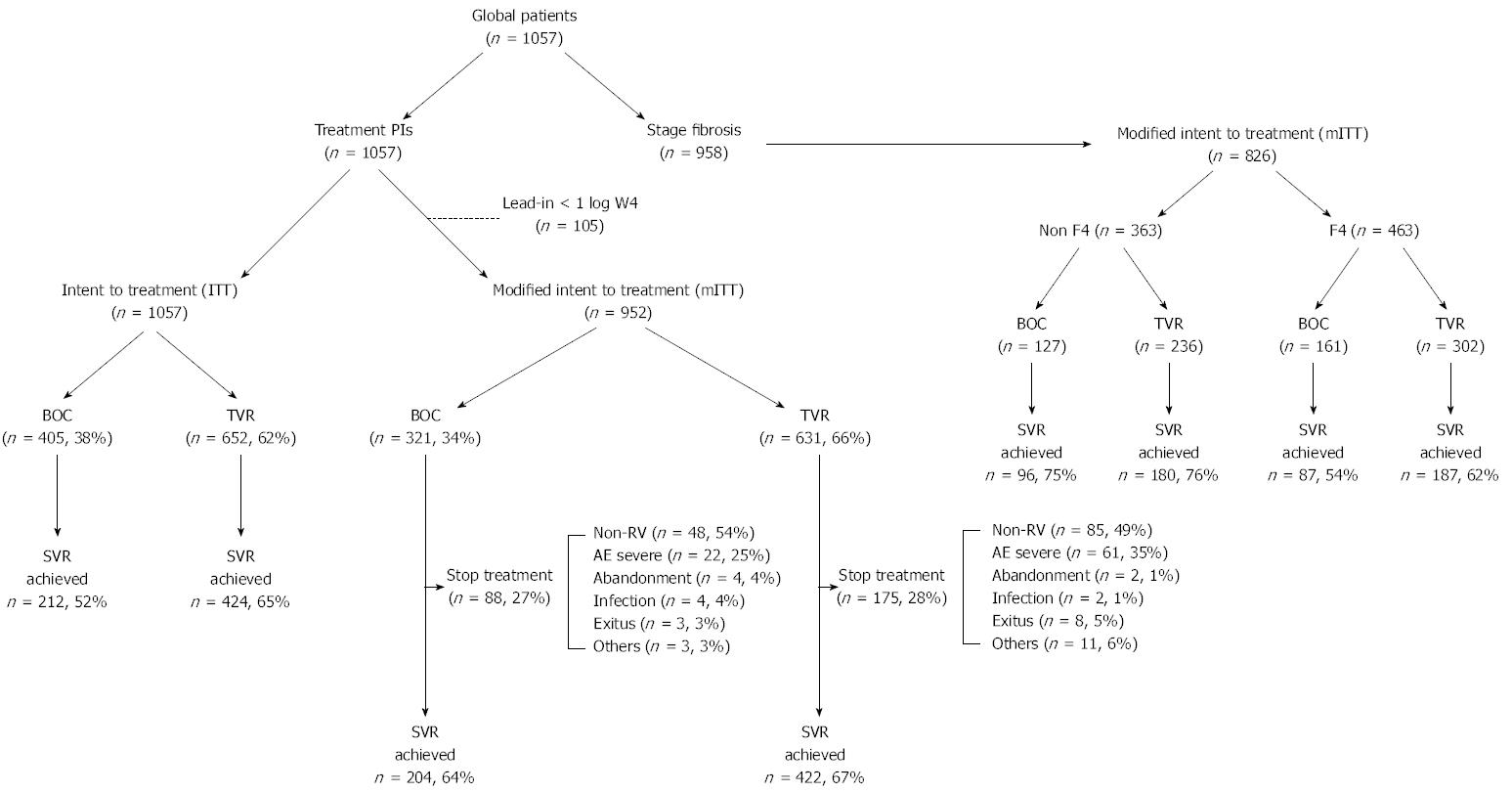
Figure 1 Study ALHAMBRA cohort.
Cohort global of patients included in the study (n = 1057) according to treatment PIs (BOC or TVR) by ITT and mITT and stage of fibrosis (non-F4 and F4). PIs: Protease inhibitors; BOC: Boceprevir; TVR: Telaprevir; ITT: Intention-to-treatment; mITT: Modified intention-to-treatment.
- Citation: Salmerón J, Vinaixa C, Berenguer R, Pascasio JM, Sánchez Ruano JJ, Serra M&, Gila A, Diago M, Romero-Gómez M, Navarro JM, Testillano M, Fernández C, Espinosa D, Carmona I, Pons JA, Jorquera F, Rodriguez FJ, Pérez R, Montero JL, Granados R, Fernández M, Martín AB, Muñoz de Rueda P, Quiles R, Alhambra Spanish Study Group. Effectiveness and safety of first-generation protease inhibitors in clinical practice: Hepatitis C virus patients with advanced fibrosis. World J Gastroenterol 2015; 21(30): 9163-9174
- URL: https://www.wjgnet.com/1007-9327/full/v21/i30/9163.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i30.9163