Copyright
©The Author(s) 2015.
World J Gastroenterol. Aug 14, 2015; 21(30): 9093-9102
Published online Aug 14, 2015. doi: 10.3748/wjg.v21.i30.9093
Published online Aug 14, 2015. doi: 10.3748/wjg.v21.i30.9093
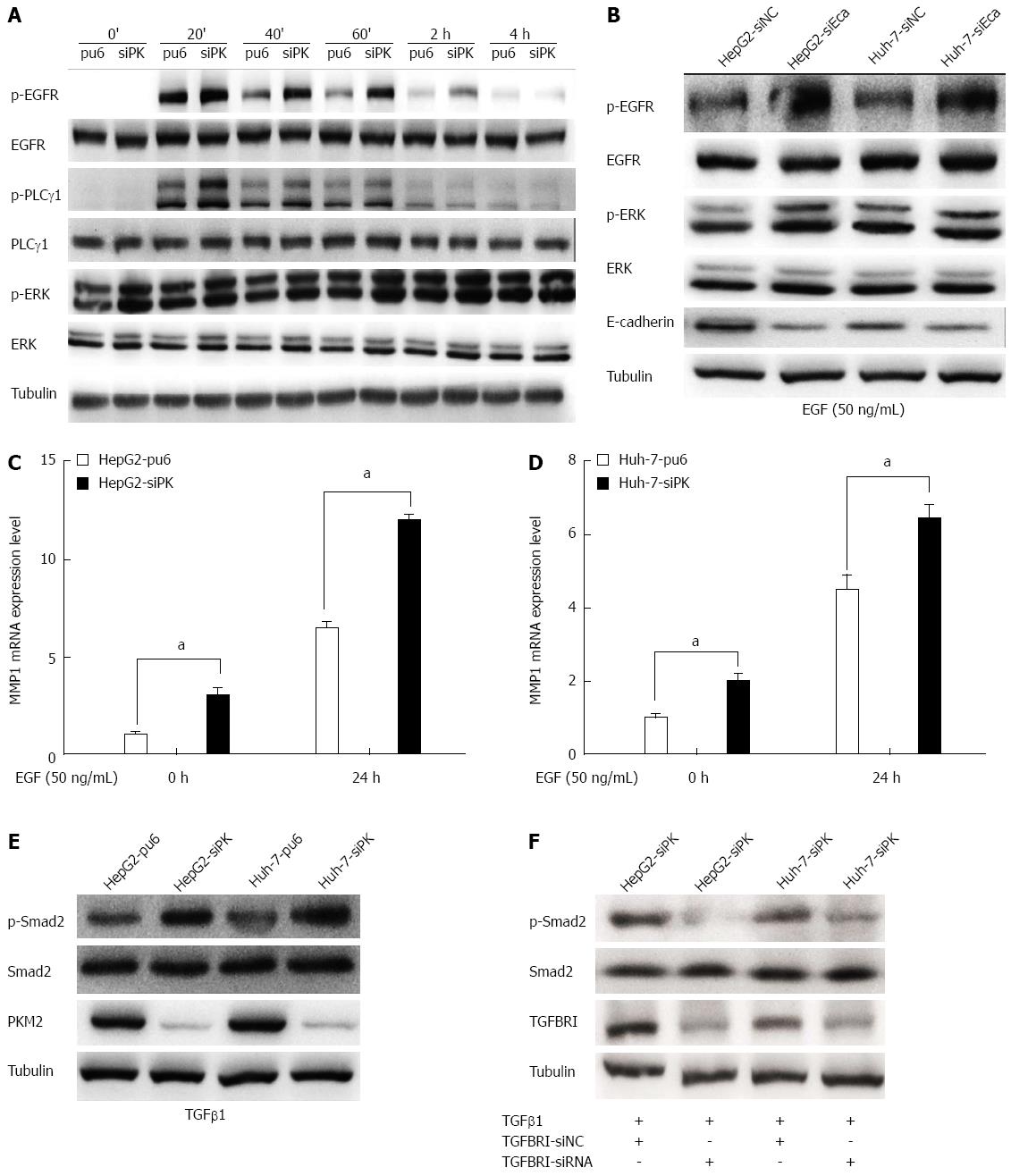
Figure 4 Depletion of pyruvate kinase M2 enhanced the activities of the epidermal growth factor/EGFR and transforming growth factor beta 1/TGFBR downstream signaling pathways.
A: Stable HepG2 and Huh-7 cells were exposed to epidermal growth factor (EGF) (50 ng/mL) for different times. Western blots of the cell lysates are shown. The protein levels of phospho-EGFR (Tyr1068), phospho-PLCγ1 (Tyr783), and phospho-ERK1/2 (Thr202/Tyr204) are shown as indicated; B: The expression of E-cadherin was knocked down in HepG2 and Huh-7 cells by transient transfection of siRNA, and after 48 h, these cells were stimulated with EGF (50 ng/mL). The protein levels of phospho-EGFR (Tyr1068) and phospho-ERK1/2 (Thr202/Tyr204) are shown as indicated; C and D: MMP1 expression levels were analyzed by quantitative real-time PCR in HepG2 and Huh-7 stable cells. Error bars represent the mean ± SD of triplicate experiments (aP < 0.05 between groups); E: Phospho-Smad2 (Ser465/467) protein levels are shown as indicated in stable HepG2 and Huh-7 cells stimulated with TGFβ1 (20 ng/mL); F: The expression of TGFBRI was knocked down in HepG2 and Huh-7 cells by transient transfection of siRNA, and after 48 h, these cells were stimulated with transforming growth factor beta 1(TGFβ1) (20 ng/mL). The protein levels of phospho-Smad2 (Ser465/467) are shown as indicated.
- Citation: Chen YL, Song JJ, Chen XC, Xu W, Zhi Q, Liu YP, Xu HZ, Pan JS, Ren JL, Guleng B. Mechanisms of pyruvate kinase M2 isoform inhibits cell motility in hepatocellular carcinoma cells. World J Gastroenterol 2015; 21(30): 9093-9102
- URL: https://www.wjgnet.com/1007-9327/full/v21/i30/9093.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i30.9093