Copyright
©The Author(s) 2015.
World J Gastroenterol. Jul 28, 2015; 21(28): 8660-8669
Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8660
Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8660
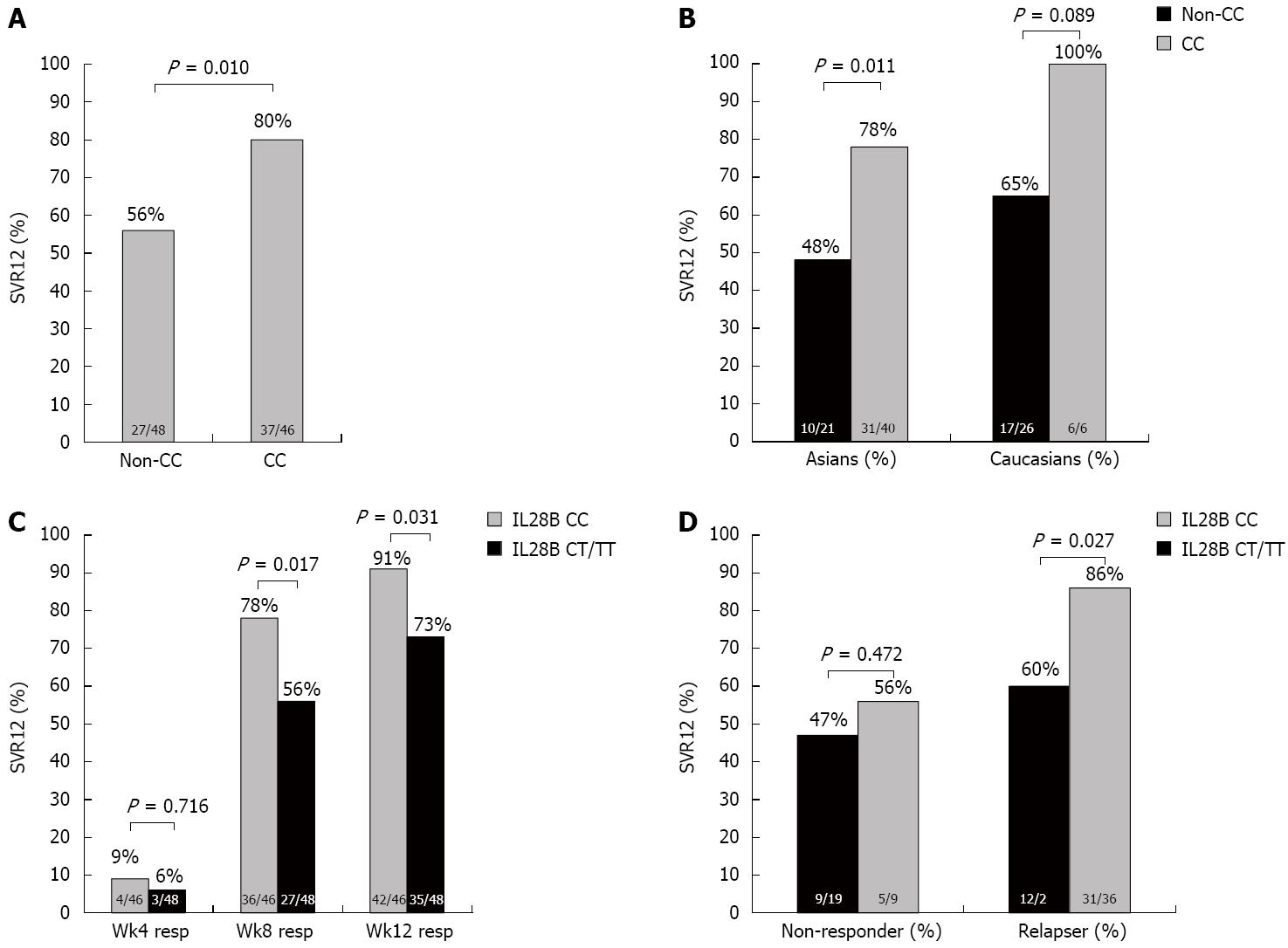
Figure 3 Sustained virological response by IL28B genotype.
A: Sustained virological response (SVR) rates by IL28B CC vs non-CC genotypes; B: SVR rates in Asians vs Caucasians by IL28B CC vs non-CC genotype; C: SVR by undetectable hepatitis C virus (HCV) RNA at treatment week 4, 8 and 12 comparing IL28B CC vs non-CC genotype; D: SVR in prior non-responders and relapsers comparing IL28B CC vs non-CC genotypes. Significant differences are shown.
- Citation: Sukeepaisarnjaroen W, Pham T, Tanwandee T, Nazareth S, Galhenage S, Mollison L, Totten L, Wigg A, Altus R, Colman A, Morales B, Mason S, Jones T, Leembruggen N, Fragomelli V, Sendall C, Guan R, Sutedja D, Tan SS, Dan YY, Lee YM, Luman W, Teo EK, Than YM, Piratvisuth T, Lim SG. Boceprevir early-access for advanced-fibrosis/cirrhosis in Asia-pacific hepatitis C virus genotype 1 non-responders/relapsers. World J Gastroenterol 2015; 21(28): 8660-8669
- URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8660.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8660