Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8723
Peer-review started: March 13, 2015
First decision: April 13, 2015
Revised: May 2, 2015
Accepted: May 27, 2015
Article in press: May 27, 2015
Published online: July 28, 2015
Processing time: 140 Days and 1.1 Hours
Esophagectomy with extended lymphadenectomy and gastric conduit reconstruction is a radical procedure for the treatment of esophageal cancer that is associated with a high morbidity rate. Gastric conduit necrosis is a fatal complication that occurs in 2% of patients. Conventionally, two-stage salvage surgery consisting of removal of the necrotic gastric conduit followed by reconstruction has been performed; however, this procedure has a high morbidity rate. We describe a 61-year-old man who underwent minimally invasive esophagectomy complicated by slowly progressive gastric conduit necrosis associated with complete neck drainage and a stable overall condition. There was a 2 cm gap in the anastomosis. Because there was no evidence of residual gastric conduit necrosis, a removable, covered self-expanding metal stent (SEMS) was inserted to bridge the anastomosis. The stent was fixed to the patient’s ear with silk thread through the lasso on its proximal end to prevent migration. Eight weeks after insertion, the stent was removed easily without any associated complications. The anastomotic defect was completely bridged with granulation tissue, showing progressive epithelialization without leakage or stenosis. The patient was discharged home in good general health. This is the first report of the successful conservative management of esophago-gastric conduit anastomosis disruption with SEMS placement.
Core tip: Gastric conduit necrosis is a fatal complication after esophagectomy. Conventionally, two-stage salvage surgery consisting of removal of the necrotic gastric conduit followed by reconstruction has been performed, but it has a high morbidity rate. On the other hand, a covered self-expanding metal stent (SEMS) has been reported to be effective for the treatment of anastomotic leakage. This is the first report of the successful conservative management of gastric conduit necrosis with SEMS placement. This case highlights the diagnosis and evaluation of the state of esophago-gastric conduit anastomosis disruption and demonstrates that a conservative approach with no surgery could result in a successful outcome.
- Citation: Oshikiri T, Yamamoto Y, Miki I, Tsuda M, Nakamura T, Fujino Y, Tominaga M, Kakeji Y. Conservative reconstruction using stents as salvage therapy for disruption of esophago-gastric anastomosis. World J Gastroenterol 2015; 21(28): 8723-8729
- URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8723.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8723
In Japan, esophagectomy with extended lymphadenectomy, gastric conduit reconstruction, and cervical anastomosis improves the prognosis of patients with esophageal cancer[1], but it is associated with high rates of morbidity and mortality[2]. Cervical anastomotic leakage after esophagectomy has been reported in 5%-24% of patients[3,4], but most cases are successfully treated with conservative approaches. On the other hand, gastric conduit necrosis following esophagectomy is a rare but critical complication[5]. Conventionally, two-stage salvage surgery consisting of the removal of the necrotic gastric conduit followed by reconstruction has been performed. However, surgical morbidity associated with this procedure is high[5].
We present a particularly rare case, in which gastric conduit necrosis and disruption of the anastomosis occurred very gradually in a patient in relatively stable condition. We discuss the presentation and successful conservative management with temporary placement of a removable covered self-expanding metal stent (SEMS).
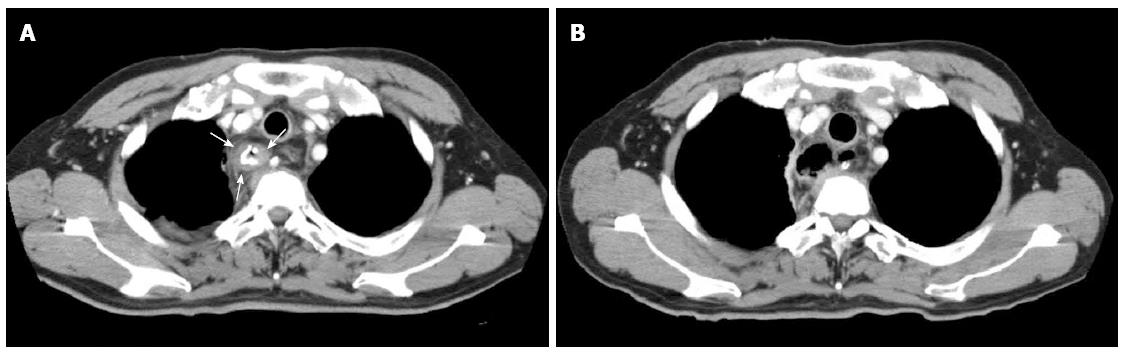
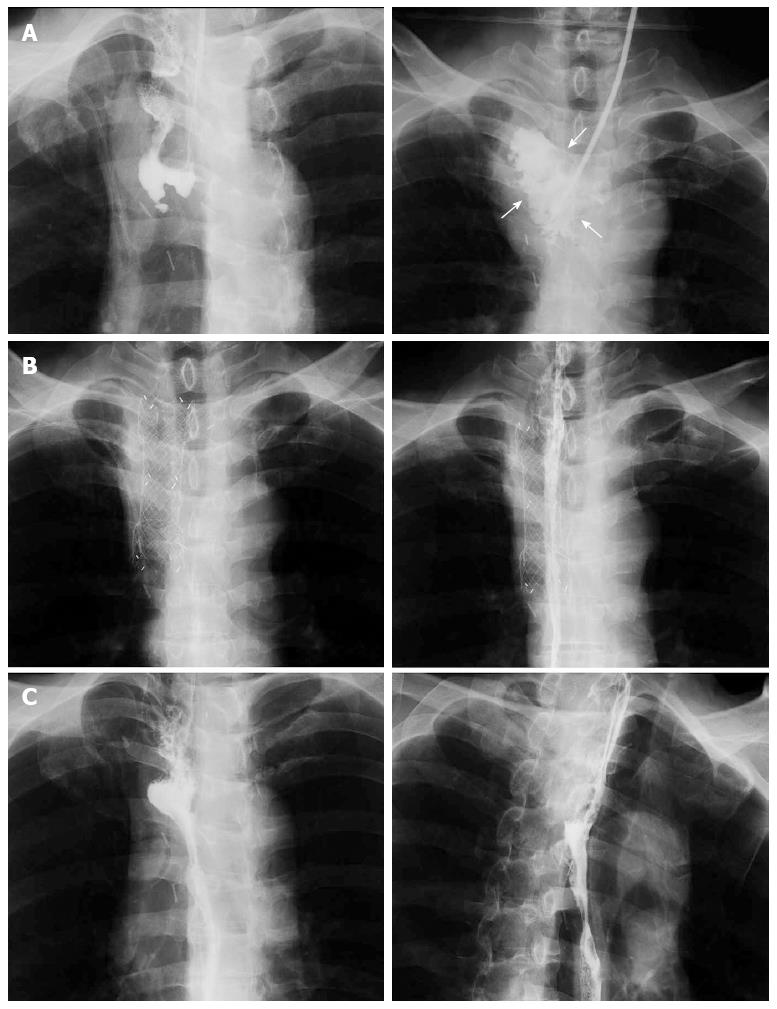
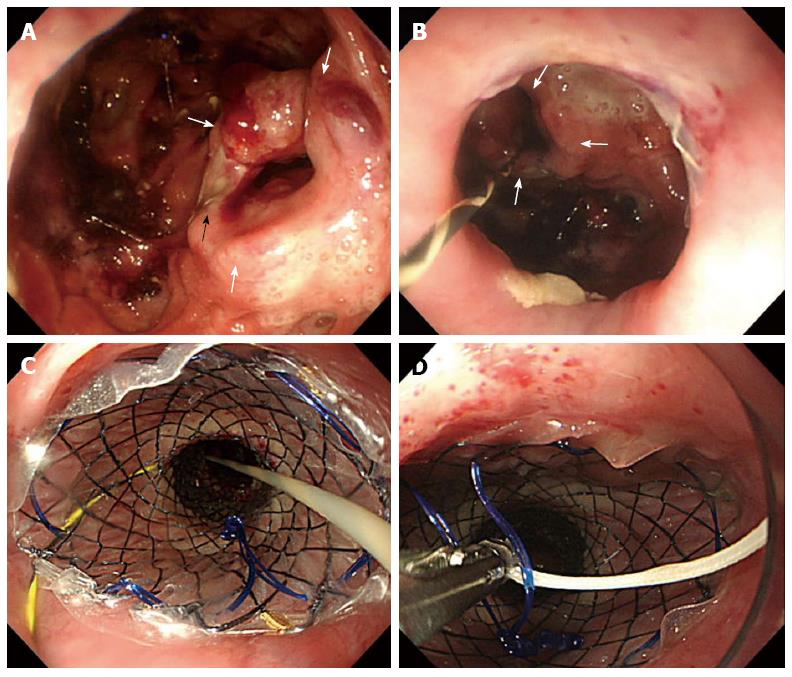
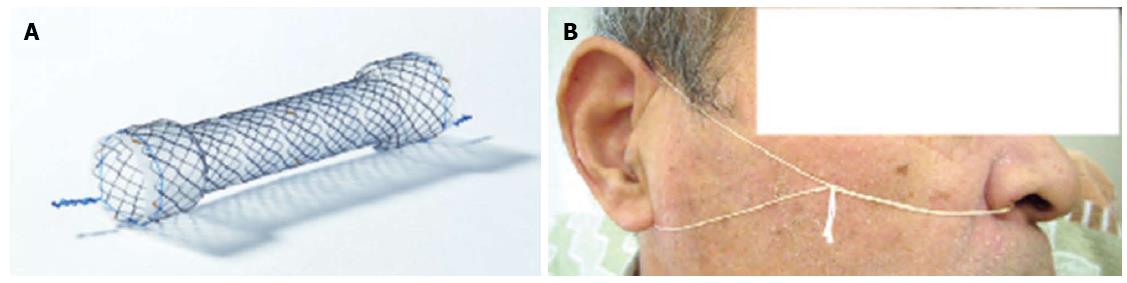
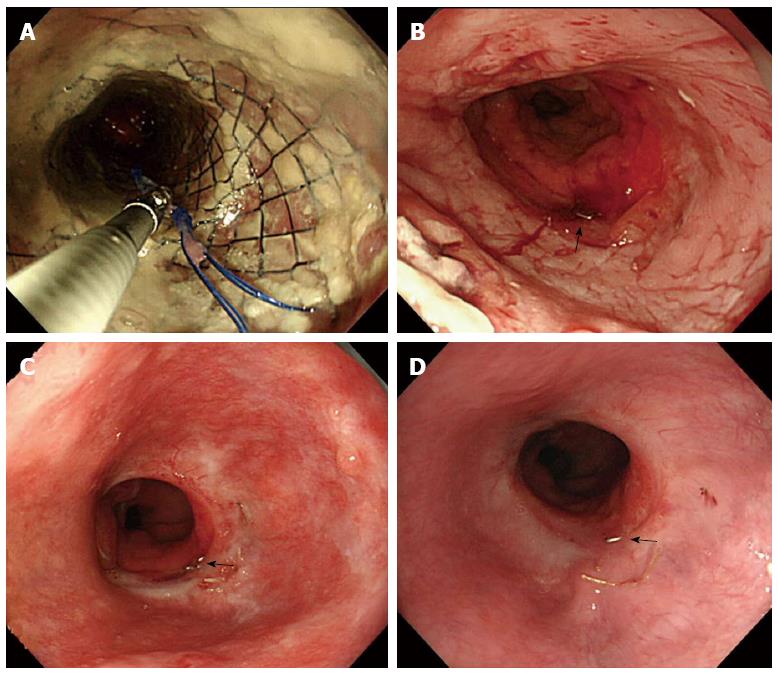
A 61-year-old man was admitted to our hospital with squamous cell carcinoma in the lower third of the esophagus, with a preoperative TNM classification[6] of T2N1M0 stage IIB. Neoadjuvant therapy was administered. The patient underwent minimally invasive esophagectomy in the prone position with two-field lymph node dissection. A gastric conduit was created by hand-assisted laparoscopic surgery in the posterior mediastinum, with a cervical anastomosis created by end-to-end stapling. There was no evidence of anastomotic tension or venous stasis of the gastric conduit. The esophago-gastric anastomotic site was ultimately determined to be located in the mediastinum (behind the manubrium). On postoperative day (POD) 6, the white blood cell (WBC) count and C-reactive protein (CRP) level were elevated at 11600/μL and 9.4 mg/dL, respectively. The neck drainage tube was dirty, with signs of anastomotic leakage. Computed tomography (CT) performed at this time showed continuity of the anastomosis, with no fluid or air surrounding it (Figure 1A, arrows). The patient was managed conservatively with a neck drainage tube and antibiotics because his general condition was stable. On POD 15, a small amount of pus was found in the drainage tube in the neck. On POD 21, the WBC and CRP levels returned to normal at 8300/μL and 1.5 mg/dL, respectively. Esophagraphy was performed to evaluate the state of the anastomosis. The gastric conduit was not imaged with oral contrast medium, and the entire volume of the contrast medium leaked out into the mediastinal cavity (Figure 2A, arrows). CT findings showed disruption of the anastomosis and mediastinal cavity (Figure 1B). Gastrointestinal endoscopy showed disruption of the anastomosis. The healthy gastric conduit was observed to contain a stump in the mediastinal cavity (thick white arrows). The staple in the stump of the gastric conduit was also observed (Figure 3A, thin black arrow). The distance between the esophageal stump and gastric conduit stump (white thick arrows) was 2 cm (Figure 3B). We diagnosed the patient with gastric conduit necrosis, which was 2 cm in length at the top of conduit and had occurred quite slowly; thus, saliva drained entirely via the neck, and the patient’s general condition remained stable. No inflammatory reaction was observed. We continued with conservative management because the patient refused surgical therapy and there was no evidence of residual gastric conduit necrosis. A removable covered SEMS, a Hanarostent Fully Covered Esophageal Stent, which was 24/18 mm in diameter and 10 cm in length (M.i.tech Co., Pyeongtaek, South Korea), was inserted to bridge the anastomosis (Figures 3B, 3C and 4A). To prevent migration of the Hanarostent, we passed silk thread through the lasso on its proximal end (Figure 3D), looped this thread, and hung it on the patient’s ear (Figure 4B). SEMS placement resulted in complete resolution of the leakage, as demonstrated by a contrast study (Figure 2B). Migration was not observed while the stent was in place. On post-stenting day (PSD) 42, gastrointestinal endoscopy was performed to evaluate recovery of the anastomotic defect through the transparent side wall of the Hanarostent. The anastomotic defect was barely detectable, and the Hanarostent maintained mobility; thus, continuation of the stenting was selected. On PSD 58, the Hanarostent was removed easily using the retrieval lasso located on its proximal end (Figure 5A). The anastomotic defect, which had originally extended over 2 cm, was completely reconstructed by the granulation tissue that had grown around the Hanarostent. The staple in the stump of the gastric conduit was confirmed (Figure 5B, thin black arrow). Complete flow of the contrast medium without leakage or stenosis was observed, as demonstrated in the contrast study (Figure 2C). On PSD 65 (Figure 5C) and PSD 84 (Figure 5D), gastrointestinal endoscopy showed chronological progression of epithelialization at the new anastomosis site, which consisted of granulation tissue. A staple was observed in the stump of the gastric conduit. The patient was able to resume a solid diet at 8 d after stent extraction and was discharged home at 17 days after extraction in good general health.
The stomach is the preferred organ for reconstruction following esophageal resection for malignant disease, but there is a 2% failure rate due to ischemia[5]. In our patient, we diagnosed gastric conduit necrosis comprehensively by a chronological change in the CT findings, the nature of the drainage, including pus, and gastrointestinal endoscopy. The CT findings on POD 6 showed continuity of the anastomosis and no evidence of malformation of the staple line; thus, anastomotic dehiscence was not caused by technical failure. Gastrointestinal endoscopy on POD 21 showed disruption of the anastomosis, but the stump of the residual gastric conduit was quite healthy without necrosis, which may have been because the ischemic area was extremely localized at the top of the gastric conduit and completely isolated, with drainage via a neck tube.
Gastric conduit necrosis is associated with a high mortality rate, although appropriate surgical management with removal of the conduit can be life-saving[5]. In our case, we did not object to early surgery as the standard of care, given the utmost importance of reducing mortality. Fortunately, there was no urgency in our patient because his gastric conduit necrosis progressed quite slowly, and his clinical condition remained stable.
Reconstructive surgery after gastric conduit necrosis has been reported to be challenging[5,7]. Dowson et al[5] have reported surgical management with removal of a conduit following gastric conduit necrosis. In their patients, reconstruction with the jejunum or left colon was performed with 0% mortality and 57% morbidity. These procedures can provide good clinical and functional outcomes, but they require a specialized multidisciplinary approach and are associated with a high morbidity rate.
To avoid surgery, we based our treatment approach on conservative therapy for the anastomotic leakage. The feasibility, effectiveness, and low morbidity of stent implantation have been reported for thoracic anastomotic leakage after esophagectomy[8]. Gastro-tracheobronchial fistula (GTF), which is rarely caused by leakage from the esophagogastrostomy site and subsequent postoperative development of a mediastinal abscess, is usually fatal[9]. Some investigators have reported that stent placement is also useful for treating GTF[10,11]. Although stent placement is a minimally invasive procedure, it is associated with severe complications, including hemorrhage, incomplete sealing, perforation, and stent migration[8,12]. Stent migration requires stent repositioning or placement of a second stent. Our method of fixing the stent to the patient’s ear with a silk thread through the lasso is considered to be reasonable and effective to prevent stent migration. Concerning complications due to stent removal, removal of the prosthesis within 6-8 wk of insertion is recommended without compromising treatment efficacy[12,13]. In our patient, gastrointestinal endoscopy revealed that the anastomotic defect was barely detectable, and the Hanarostent maintained mobility at 6 wk after insertion. Therefore, the duration of stent therapy exceeded 8 wk to ideally reconstruct the anastomosis, which was completely disrupted. There were no complications associated with stent removal.
Recently, a new approach for the treatment of anastomotic leakage following esophageal resection has been reported involving the combination of vacuum-assisted therapy with covered SEMS[14]. In this approach, an Endo-sponge (Braun BBD Aesculap GmbH, Tuttlingen, Germany) is placed into the esophageal lumen and pushed into the para esophageal cavity, which is overstented with a partially covered SEMS. The suction tube of the sponge is retrieved through the nose and connected to a continuous negative pressure of 75 to 100 mmHg within 18 h in a stepwise manner, and the pressure is increased when secretion production is diminished[14]. In our patient, the paraesophageal cavity was too large to enclose the sponge tightly. Additionally, he experienced complete drainage by the percutaneous drain and thus, simple SEMS therapy was selected. In the case of leakage in a patient with a small paraesophageal cavity without a percutaneous drainage tube, endoluminal vacuum therapy with an Endo-sponge is an ideal and excellent therapy.
To the best of our knowledge, this is the first report of the successful conservative management of gastric conduit necrosis as a complication of esophagectomy. Our experience suggests that in very carefully selected patients in whom the progression of gastric conduit ischemia is slow, there is no associated inflammation of the mediastinum, and the general condition is stable without evidence of sepsis, a trial of conservative management may be effective. By contrast, a patient with early onset gastric conduit ischemia that occurs within POD 5 may not be suitable for stent therapy because the progression will likely be acute and critical. To diagnose conduit necrosis immediately, rapid execution of gastrointestinal endoscopy should be recommended if there are any signs of anastomotic leakage. Considering the abovementioned criteria, the distance between the two ends of the anastomosis or the circumference of the leakage may not be the limit for stent therapy.
In conclusion, this case highlights the diagnosis and evaluation of esophago-gastric conduit anastomosis disruption and demonstrates that a conservative approach with no surgery may result in a successful outcome.
A 61-year-old man with esophageal cancer underwent minimally invasive esophagectomy with cervical esophago-gastric anastomosis in the prone position.
Disruption of the anastomosis with gastric conduit necrosis.
Simple anastomotic leakage.
WBC: 11600/μL; CRP: 9.4 mg/dL; and all other laboratory parameters were within the normal limits.
Computed tomography findings and gastrointestinal endoscopy showed disruption of the anastomosis.
A removable, self-expanding metal stent (SEMS) was inserted to bridge the anastomosis.
The feasibility, effectiveness, and low morbidity of stent implantation for the treatment of simple anastomotic leakage have been reported, but there are few reports of the successful conservative management of anastomosis disruption with SEMS placement.
Gastric conduit necrosis following esophagectomy occurs in 2% of patients.
The authors recommend that SEMS therapy for anastomosis disruption when the progression of gastric conduit ischemia is slow, there is no associated inflammation of the mediastinum, and the patient’s general condition is stable without evidence of sepsis. The distance between the two ends of the anastomosis or the circumference of the leakage may not be the limit for stent therapy.
This article is a case report about the conservative management of gastric tube necrosis as a complication of esophagectomy. The authors describe a 61-year-old man who underwent minimally invasive esophagectomy complicated by slowly progressive gastric conduit necrosis associated with complete neck drainage and stable overall condition. The patient’s anastomotic leakage was treated by inserting a covered self-expanding metal stent into the esophagus.
P- Reviewer: Bogoevski D, Boukerrouche A, Kosugi S S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Akiyama H, Tsurumaru M, Udagawa H, Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg. 1994;220:364-372; discussion 372-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 691] [Cited by in F6Publishing: 620] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 2. | Watson A. Operable esophageal cancer: current results from the West. World J Surg. 1994;18:361-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | van Rossum PS, Haverkamp L, Verkooijen HM, van Leeuwen MS, van Hillegersberg R, Ruurda JP. Calcification of arteries supplying the gastric tube: a new risk factor for anastomotic leakage after esophageal surgery. Radiology. 2015;274:124-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Yamasaki M, Miyata H, Yasuda T, Shiraishi O, Takahashi T, Motoori M, Yano M, Shiozaki H, Mori M, Doki Y. Impact of the route of reconstruction on post-operative morbidity and malnutrition after esophagectomy: a multicenter cohort study. World J Surg. 2015;39:433-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Dowson HM, Strauss D, Ng R, Mason R. The acute management and surgical reconstruction following failed esophagectomy in malignant disease of the esophagus. Dis Esophagus. 2007;20:135-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721-1724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 571] [Cited by in F6Publishing: 631] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 7. | Umezawa H, Matsutani T, Ogawa R, Hyakusoku H. Immediate free jejunum transfer for salvage surgery of gastric tube necrosis. Case Rep Gastrointest Med. 2014;2014:327549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Kauer WK, Stein HJ, Dittler HJ, Siewert JR. Stent implantation as a treatment option in patients with thoracic anastomotic leaks after esophagectomy. Surg Endosc. 2008;22:50-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Brega Massone PP, Infante M, Valente M, Conti B, Carboni U, Cataldo I. Gastrobronchial fistula repair followed by esophageal leak--rescue by transesophageal drainage of the pleural cavity. Thorac Cardiovasc Surg. 2002;50:113-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Bona D, Sarli D, Saino G, Quarenghi M, Bonavina L. Successful conservative management of benign gastro-bronchial fistula after intrathoracic esophagogastrostomy. Ann Thorac Surg. 2007;84:1036-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Martin-Smith JD, Larkin JO, O’Connell F, Ravi N, Reynolds JV. Management of gastro-bronchial fistula complicating a subtotal esophagectomy: a case report. BMC Surg. 2009;9:20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | van Heel NC, Haringsma J, Spaander MC, Bruno MJ, Kuipers EJ. Short-term esophageal stenting in the management of benign perforations. Am J Gastroenterol. 2010;105:1515-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Song HY, Park SI, Do YS, Yoon HK, Sung KB, Sohn KH, Min YI. Expandable metallic stent placement in patients with benign esophageal strictures: results of long-term follow-up. Radiology. 1997;203:131-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Gubler C, Schneider PM, Bauerfeind P. Complex anastomotic leaks following esophageal resections: the new stent over sponge (SOS) approach. Dis Esophagus. 2013;26:598-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |