Published online Jul 21, 2015. doi: 10.3748/wjg.v21.i27.8425
Peer-review started: February 10, 2015
First decision: March 10, 2015
Revised: March 17, 2015
Accepted: April 17, 2015
Article in press: April 17, 2015
Published online: July 21, 2015
Processing time: 173 Days and 22.9 Hours
AIM: To analyze the virtual touch tissue quantification (VTTQ) and virtual touch imaging quantification (VTIQ) techniques, and identify possible factors that may influence VTTQ and VTIQ measurements.
METHODS: One hundred and eighty-six (104 women/82 men) of 323 subjects met the inclusion criteria (age > 18 years, no history of chronic or gastrointestinal disease, body-mass index (BMI) < 30 kg/m², a fasting period of at least three hours, no history of hepatotoxic pharmaceuticals, alcohol consumption < 24 g/d in men and < 12 g/d in women, and normal findings upon ultrasound examination of the abdomen). Measurements were taken at depths of 50 mm with VTTQ, 15 mm and 25 mm with VTIQ in the right hepatic lobe, and at 15 mm with only VTIQ in the left hepatic lobe. The examiner acquired six measurements per position, thereby giving 24 measurements in total.
RESULTS: The 95% confidence intervals of mean were 1.23-1.29 m/s for VTTQ and 1.29-1.37 m/s, 1.17-1.23 m/s, and 1.48-1.57 m/s for VTIQ in a depth of 15 mm and 25 mm in the right hepatic lobe and 15 mm in the left hepatic lobe. Only superficial measurements in the right hepatic lobe with the VTIQ method exhibited an effect of age on shear wave velocity. Measurements acquired using the 6C1 probe with the VTTQ method showed no dependence on BMI. By comparison, BMI influenced measurements taken with the VTIQ method using the 9L4 probe in the superficial and deep areas of the right hepatic lobe, as well as in the left hepatic lobe (P = 0.0160, P = 0.0019, P = 0.0173, respectively). Gender influenced measurements at depths of 50 mm with VTTQ and 25 mm with VTIQ in the right hepatic lobe (P = 0.0001, P = 0.0269). Significant differences were found between measurements with the 6C1 (VTTQ) and 9L4 probes (VTIQ) (P = 0.0067), between superficial and deep measurements (P < 0.0001), and between the right and left lobes of the liver (P < 0.0001).
CONCLUSION: Measurements in the right lobe and deep regions are preferable. Gender differences must be considered. BMI must be considered when assessing VTIQ technology.
Core tip: Virtual touch tissue quantification (VTTQ) and virtual touch imaging and quantification (VTIQ) are two new elastographic techniques for estimating tissue stiffness. Both methods (VTTQ and VTIQ) allow a quantitative and non-invasive assessment of shear wave velocity. Shear wave speed quantification may be a diagnostic tool in the diagnosis of fibrotic liver changes. Therefore, standard values in healthy liver tissue must be generated. The objective of the present study is to analyze the VTTQ and VTIQ techniques. Possible factors that may influence VTTQ and VTIQ measurements were also studied.
- Citation: Galgenmueller S, Jaeger H, Kratzer W, Schmidt SA, Oeztuerk S, Haenle MM, Mason RA, Graeter T. Parameters affecting different acoustic radiation force impulse applications in the diagnosis of fibrotic liver changes. World J Gastroenterol 2015; 21(27): 8425-8432
- URL: https://www.wjgnet.com/1007-9327/full/v21/i27/8425.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i27.8425
A variety of elastographic techniques have been developed to facilitate the non-invasive assessment of tissue properties. These techniques fall into the categories of “strain imaging”, “shear wave speed measurement”, and “shear wave speed imaging”[1]. The advantage of the latter two categories lies in the quantitative assessment of shear wave velocity (SWV). A few studies have investigated the application of acoustic radiation force impulse (ARFI) shear wave speed quantification in the diagnosis of fibrotic liver changes and, in some cases, compared its findings with those of other diagnostic techniques, such as biopsy, serum liver function tests (LFTs), and transient elastography[2-4]. A meta-analysis of studies using liver biopsy as the reference quantified this ARFI method’s diagnostic as 0.87 Area under Receiver Operating Characteristic Curve (AUROC) for the evaluation of significant liver fibrosis and 0.93 (AUROC) for the diagnosis of cirrhosis[5]. In addition, these studies have documented a good degree of reproducibility for these techniques[6,7]. Certain studies have established normal reference values for the Virtual Touch Tissue Quantification (VTTQ) technique using the Acuson S2000 in healthy children[8-10] and adults[7,11-20]. To date, use of the new ARFI technique of Virtual Touch Imaging Quantification (VTIQ) has only been described for normal mammary tissue[21].
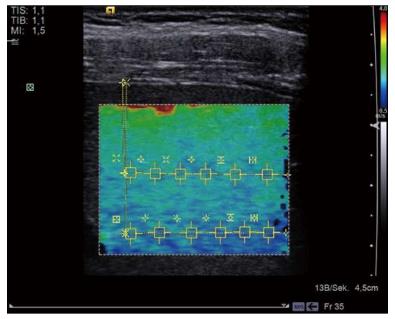
Earlier elastographic techniques, such as quasi-static elastography, depend on a manual compression of the tissue to arrive at estimates of tissue stiffness[22]. New methods based on acoustic radiation force (ARF) permit such evaluation without the reliance on examiner-dependent pressure on the tissue. A shear wave perpendicular to the direction of propagation is produced by means of a brief high-intensity acoustic impulse. This wave is localized using tracking beams and a quantitative value in m/s is calculated[1,23]. The VTTQ technique permits the selection of an area of liver tissue, as defined in a region of interest (ROI), allowing measurements to a depth of up to 8.0 cm. The VTIQ method uses a moveable Q box quantification tool to select a defined region. A pulse sequence of 256 acquisition beam lines covers a width of 38 mm. Point shear wave elastography using the VTTQ method is performed in each of these regions[1]. Stiffer regions appear red, while softer regions appear blue in the resulting qualitative color elastogram. Using an ROI, a quantitative SWV in m/s can be measured at selected points (Figure 1).
The objective of the present study is to analyze the VTTQ and VTIQ techniques, and assess the potential effects of factors such as age, gender, body-mass index (BMI), fasting time, use of oral contraceptive steroids, depth and position of measurement, and choice of probe.
Taking part in the study were 323 subjects, 186 of whom met the inclusion criteria. Inclusion criteria included age > 18 years, no history of chronic or gastrointestinal disease, BMI < 30 kg/m2, a fasting period of at least three hours, no history of hepatotoxic pharmaceuticals, alcohol consumption < 24 g/d in men and < 12 g/d in women, and normal findings upon ultrasound examination of the abdomen. The study was approved by the institutional ethics commission (Nr. 397/13) and subjects provided their written informed consent.
Nine examiners performed ultrasound examinations using the convex (6C1 HD, 1.5-5.5 MHz) and linear probes (9L4, 4.0-9.0 MHz) of the Acuson S3000 (Siemens Medical Solutions, Mountain View, CA, United States). Measurements of the right hepatic lobe were performed using the VTTQ (6C1-probe) and VTIQ (9L4-probe) method. Subjects were placed in a supine position with an elevated right arm and suppressed respiration at breathing baseline. The probe was placed over the sixth or seventh intercostal space. Examiners initiated VTIQ measurement at a depth adjustment of 4.5 cm with minimum and maximum shear wave velocities of 0.5 m/s and 4.0 m/s, respectively. VTTQ measurements were performed at a depth of 5.0 cm beneath the skin surface, while VTIQ measurements were performed at 15 and 25 mm from the level of the liver capsule. In addition, a measurement at 15 mm was obtained in the left hepatic lobe with VTIQ. The 9L4 probe was therefore located beneath the xiphoid process in the cross section. A total of six measurements were acquired per position.
Statistical analysis was performed using the SAS statistical software package (version 9.2, Cary, North Carolina). Results were presented as mean ± SD, median, and range for continuous variables. Normal distribution was tested using the Shapiro-Wilk test. Differences between two groups were identified using the t-test or Mann-Whitney U test. A one-way analysis of variance or Kruskal-Wallis test was performed to compare more than two groups. The relationship between SWV and continuous variables were investigated using Spearman’s correlation coefficient (r). All tests were two-sided.
A total of 186 subjects were enrolled in the study (104 women and 82 men). The mean age was 31.05 ± 12.93 years. A statistically significant difference was identified between women and men for BMI (P < 0.0001; Table 1). One subject underwent post-menopausal hormone replacement therapy and used a copper-based intrauterine device. Three subjects took an iron preparation. Three further volunteers had in the past taken L-thyroxine.
| mean ± STD (Range) | P value | |||
| Women | Men | All | ||
| (n = 104; 55.9%) | (n = 82; 44.1%) | (n = 186) | ||
| Age (yr) | 31.94 ± 13.46 | 29.93 ± 12.22 | 31.05 ± 12.93 | 0.6743 |
| (18.00-71.00) | (18.00-82.00) | (18.00-82.00) | ||
| BMI (kg/m2) | 21.52 ± 2.02 | 22.98 ± 2.01 | 22.16 ± 2.14 | < 0.0001 |
| (17.57-28.58) | (18.52-27.76) | (17.57-28.58) | ||
| Liver size (mm) | 142.31 ± 14.75 | 144.44 ± 13.17 | 143.25 ± 14.07 | 0.3055 |
| (106.00-172.00) | (116.00-177.00) | (106.00-177.00) | ||
A significant correlation was not identified between age and SWV with the 6C1 or 9L4 probes at all depths (P > 0.05). After classification of age into four classes (n = 69, n = 57, n = 39, n = 21), only in the case of measurements at a depth of 15 mm in the right hepatic lobe was there a significant difference between the age groups (P = 0.0090; Table 2). The mean and standard deviation in this case stood at 1.29 ± 0.24 m/s, 1.25 ± 0.25 m/s, 1.38 ± 0.32 m/s, and 1.57 ± 0.44 m/s for the individual age classes, respectively. The SWVs for the 6C1 probe and other depths with the 9L4 probe are given in Table 3.
| P value | ||||
| 6C1-50 mmR | 9L4-15 mmR | 9L4-25 mmR | 9L4-15mmL | |
| Age groups | 0.1384 | 0.0090 | 0.6286 | 0.2517 |
| BMI groups | 0.1166 | 0.0160 | 0.0019 | 0.0173 |
| Oral contraceptives | 0.6415 | 0.0990 | 0.8771 | 0.0461 |
| Gender | 0.0001 | 0.5425 | 0.0269 | 0.9532 |
| 9L4-15 mmR | 0.0901 | - | - | - |
| 9L4-25 mmR | 0.0067 | < 0.0001 | - | - |
| 9L4-15 mmL | < 0.0001 | < 0.0001 | < 0.0001 | - |
| mean ± STD or median (range) (m/s) | |||||
| 6C1-50 mmR | 9L4-15 mmR | 9L4-25 mmR | 9L4-15 mmL | ||
| Age group (yr) | 18-23 (n = 69) | 1.28 ± 0.22 | 1.29 ± 0.24 | 1.19 ± 0.20 | 1.51 ± 0.27 |
| 1.23 (0.95-1.91) | 1.27 (0.81-1.82) | 1.18 (0.64-1.68) | 1.50 (0.79-2.31) | ||
| 24-29 (n = 57) | 1.28 ± 0.20 | 1.25 ± 0.25 | 1.22 ± 0.19 | 1.53 ± 0.27 | |
| 1.26 (0.97-1.97) | 1.22 (0.88-1.79) | 1.23 (0.77-1.59) | 1.52 (0.83-2.12) | ||
| 30-50 (n = 39) | 1.25 ± 0.18 | 1.38 ± 0.32 | 1.22 ± 0.23 | 1.48 ± 0.35 | |
| 1.21 (0.98-1.75) | 1.29 (0.88-2.30) | 1.18 (0.82-1.83) | 1.46 (0.90-2.67) | ||
| > 50 (n = 21) | 1.19 ± 0.20 | 1.57 ± 0.44 | 1.16 ± 0.25 | 1.64 ± 0.33 | |
| 1.12 (1.00-1.85) | 1.55 (1.03-2.57) | 1.17 (0.72-1.73) | 1.65 (0.99-2.31) | ||
| BMI group (kg/m2) | < 20.00 (n = 30) | 1.35 ± 0.25 | 1.35 ± 0.24 | 1.30 ± 0.19 | 1.57 ± 0.24 |
| 1.29 (0.98-1.97) | 1.39 (0.94-1.82) | 1.29 (0.85-1.68) | 1.59 (1.00-2.12) | ||
| 20.00-20.99 (n = 30) | 1.20 ± 0.15 | 1.34 ± 0.28 | 1.28 ± 0.23 | 1.60 ± 0.29 | |
| 1.21 (0.97-1.60) | 1.34 (0.88-2.01) | 1.20 (0.95-1.83) | 1.51 (1.00-2.24) | ||
| 21.00-21.99 (n = 36) | 1.21 ± 0.18 | 1.23 ± 0.22 | 1.21 ± 0.20 | 1.50 ± 0.31 | |
| 1.16 (0.97-1.65) | 1.19 (0.89-1.72) | 1.15 (0.98-1.67) | 1.47 (0.83-2.67) | ||
| 22.00-22.99 (n = 28) | 1.29 ± 0.22 | 1.25 ± 0.23 | 1.18 ± 0.20 | 1.59 ± 0.32 | |
| 1.23 (1.06-1.84) | 1.25 (0.81-1.71) | 1.20 (0.80-1.47) | 1.59 (1.14-2.31) | ||
| 23.00-23.99 (n = 24) | 1.25 ± 0.15 | 1.30 ± 0.36 | 1.15 ± 0.19 | 1.34 ± 0.27 | |
| 1.25 (0.95-1.67) | 1.16 (0.88-2.30) | 1.18 (0.64-1.54) | 1.36 (0.79-1.77) | ||
| 24.00-25.00 (n = 20) | 1.29 ± 0.19 | 1.34 ± 0.25 | 1.10 ± 0.16 | 1.47 ± 0.28 | |
| 1.24 (1.03-1.88) | 1.31 (0.97-1.94) | 1.09 (0.77-1.43) | 1.52 (0.90-1.89) | ||
| > 25.00 (n = 18) | 1.26 ± 0.25 | 1.63 ± 0.46 | 1.12 ± 0.23 | 1.59 ± 0.29 | |
| 1.16 (1.00-1.85) | 1.56 (1.05-2.57) | 1.16 (0.72-1.50) | 1.61 (0.99-2.07) | ||
| Fasting time (h) | 3.0 (n = 54) | 1.28 ± 0.23 | 1.30 ± 0.33 | 1.21 ± 0.25 | 1.50 ± 0.33 |
| 1.22 (0.98-1.97) | 1.22 (0.81-2.56) | 1.17 (0.64-1.83) | 1.54 (0.83-2.31) | ||
| 3.5-7.0 (n = 66) | 1.22 ± 0.19 | 1.37 ± 0.28 | 1.19 ± 0.20 | 1.59 ± 0.28 | |
| 1.20 (0.97-1.91) | 1.34 (0.88-2.30) | 1.18 (0.77-1.65) | 1.51 (1.06-2.67) | ||
| ≥ 8.0 (n = 66) | 1.28 ± 0.19 | 1.32 ± 0.30 | 1.22 ± 0.20 | 1.48 ± 0.27 | |
| 1.29 (0.95-1.85) | 1.27 (0.89-2.57) | 1.21 (0.78-1.73) | 1.50 (0.79-2.07) | ||
| Oral contraceptives | No (n = 63) | 1.24 ± 0.21 | 1.38 ± 0.30 | 1.23 ± 0.21 | 1.57 ± 0.32 |
| 1.18 (0.95-1.81) | 1.40 (0.97-2.57) | 1.20 (0.80-1.73) | 1.54 (0.79-2.67) | ||
| Yes (n = 41) | 1.20 ± 0.19 | 1.28 ± 0.30 | 1.24 ± 0.20 | 1.45 ± 0.25 | |
| 1.17 (0.97-1.84) | 1.26 (0.81-2.13) | 1.22 (0.64-1.64) | 1.47 (0.83-1.98) | ||
A negative correlation was found between BMI and the measurements at 25 mm depth in the right hepatic lobe (r = -0.29910, P≤ 0.0001). No significant correlation could be detected at 5 cm, 15 mm in the right hepatic lobe and 15 mm in the left hepatic lobe (P = 0.7152, P = 0.3666, P = 0.1973). The subjects were divided into seven BMI classes containing 30, 30, 36, 28, 24, 20, and 18 volunteers, respectively. No statistically significant difference between the groups could be detected for the 6C1 probe (P = 0.1166). By contrast, the linear probe showed significant differences for all measurement positions (P = 0.0160, P = 0.0019, P = 0.0173; Table 3).
Three hours’ fasting was reported by 54 subjects, while 66 subjects each reported fasting for 3.5-7.0 or ≥ 8.0 h. No statistically significant differences between the groups could be identified for either probe at any of the measurement depths (P = 0.0665, P = 0.1603, P = 0.9511, P = 0.5788; Table 3).
Forty-one of the 104 women participating in the study (39.4%) used oral contraceptive steroids. For measurements in the right hepatic lobe, there were no statistically significant differences between those who did and did not use oral contraceptives (P = 0.6415, P = 0.0990, P = 0.8771). By contrast, a statistically significant difference between the groups was identified for examination of the left hepatic lobe (P = 0.0461): here, women using oral contraceptives showed lower SWVs (1.45 ± 0.25 m/s vs 1.57 ± 0.32 m/s; Table 3).
A significant difference between men and women was identified for the convex probe (P = 0.0001), with men exhibiting higher values. There was also a statistically significant difference with the 9L4 probe using the VTIQ technique at 25 mm (P = 0.0269), with higher values being observed in women. Neither the surface measurement at 15 mm depth of the right hepatic lobe nor the tissue measurements at 15 mm depth in the left hepatic lobe showed any statistically significant differences (P = 0.5425, P = 0.9532; Table 4).
| Women (n = 104) | Men (n = 82) | |||
| mean ± STD | median (range) | mean ± STD | median (range) | |
| 6C1-50 mmR | 1.22 (0.20) | 1.18 (0.95-1.84) | 1.31 (0.20) | 1.27 (1.03-1.97) |
| 9L4-15 mmR | 1.34 (0.30) | 1.30 (0.81-2.57) | 1.32 (0.31) | 1.24 (0.88-2.56) |
| 9L4-25 mmR | 1.23 (0.21) | 1.21 (0.64-1.83) | 1.17 (0.21) | 1.15 (0.79-1.83) |
| 9L4-15 mmL | 1.53 (0.30) | 1.50 (0.79-2.67) | 1.52 (0.29) | 1.52 (0.90-2.31) |
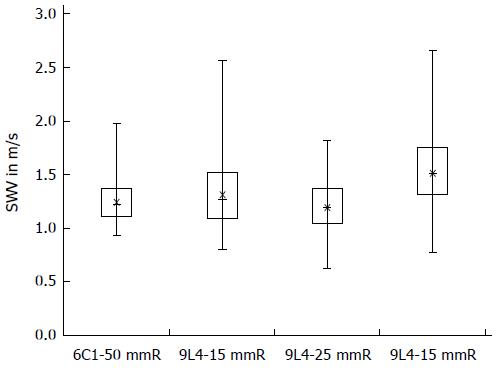
A highly significant correlation was detected for the VTTQ technique with the 6C1 probe in a depth of 5.0 cm under the skin and the VTIQ technique with the 9L4 probe at a depth of 25 mm under the liver capsule (r = 0.2838; P < 0.0001), as well as for measurements in the right and left hepatic lobes at 15 mm (r = 0.2776; P = 0.0001) with the VTIQ technique. The comparison of measurements at 15 mm using the linear probes and at 5.0 cm using the convex probe also showed a significant correlation (r = 0.24027, P = 0.0006) in the right liver lobe. There was also a correlation for measurements at both 15 mm and 25 mm in the right hepatic lobe using the VTIQ technique of the linear probe (r = 0.19166; P = 0.0088). An overview of all median values, means, standard deviations, range, and minimum and maximum measurements with the VTTQ technique of the convex probe (6C1) and the VTIQ technique of the linear probe (9L4) are given in Table 5. Figure 2 shows the box-plot diagram of measurements with the two probes.
| mean | 95%CI mean | STD | median | 95%CI median | min-max | |
| (m/s) | (m/s) | (m/s) | (m/s) | (m/s) | (m/s) | |
| 6C1-50 mmR | 1.26 | 1.23-1.29 | 0.20 | 1.22 | 1.20-1.26 | 0.95-1.97 |
| 9L4-15 mmR | 1.33 | 1.29-1.37 | 0.30 | 1.27 | 1.23-1.37 | 0.81-2.57 |
| 9L4-25 mmR | 1.20 | 1.17-1.23 | 0.21 | 1.19 | 1.17-1.23 | 0.64-1.83 |
| 9L4-15 mmL | 1.52 | 1.48-1.57 | 0.30 | 1.51 | 1.48-1.57 | 0.79-2.67 |
A total of 186 healthy volunteers were examined with the VTTQ and VTIQ techniques using the Acuson S3000 scanner. Subjects with pathological changes or tumors of the liver were excluded from the study. According to EFSUMB guidelines, the probe was positioned intercostally for measurements in the right hepatic lobe[24]. For anatomical reasons, however, examination of the left hepatic lobe required an abdominal approach. With respect to positioning the patient for examination, no consensus has been reached. While some authors preferred to examine the liver with subjects lying on their left side[6,19], others performed the examination with subjects in a supine position[15,18]. Six measurements were acquired at each of the four positions. A defined number of measurements for reliable SWV determination has yet to be established[6-8,10-16,18-20,25].
A statistically significant difference in SWV was observed between women and men in measurements with the convex probe at a depth of 5.0 cm and with the linear probe at a depth of 25 mm. Studies of transient elastography also observed an effect of gender in SWV[26]. By contrast, studies with the VTTQ technique failed to demonstrate any dependence on gender[13,14,16,18-20]. However, with the exception of two studies[16,20], measurements were taken at a depth of 1.0-2.0 cm under the capsule. The findings of the present study similarly failed to demonstrate any statistically significant difference at a depth of 15 mm. However, it must be noted that women and men included in the study collective differed significantly in terms of BMI (P < 0.0001), which can represent a source of error in calculations. A definitive statement on the dependence of SWV on gender is therefore not possible based on data from the present study.
Investigations of the influence of BMI on measurements using the VTTQ technique have reported contradictory findings[12,14,15,20]. Data from the present study concerning measurements using the 6C1 probe failed to demonstrate either a significant correlation between SWV and BMI (P = 0.7152) or statistically significant differences in SWV between the individual BMI classes (P = 0.1166). Study criteria, however, excluded subjects with a BMI > 30 kg/m² from the collective of subjects with normal values, and only 9.8% of the 186 participants exhibited a BMI > 25 kg/m². By contrast, when the 9L4 probe was used, an influence of BMI was detected at all measurement positions, and there was a tendency toward lower SWV values with higher BMI. In fact, at a depth of 25 mm, SWV’s correlation with BMI was highly significant (P < 0.0001). Compared with the 6C1 probe, the 9L4 probe exhibited a more limited penetration into liver tissue. It is unclear whether the VTIQ, with its greater flow line, is more prone to artifacts, which may result in an underestimation of SWV at the margins of the measurement. Further research is required to elucidate these questions.
Significantly lower SWV values were measured in the left hepatic lobe of women taking oral contraceptive steroids than in women not using hormonal contraception (P = 0.0461). Animal experiments have demonstrated a protective effect of female sex hormones on the formation of the extracellular matrix in the liver[27]. The relevance of these findings in humans remains unclear. Previous studies have failed to detect any influence of oral contraceptives on SWV[14]. Because of the increased scatter of values in the left hepatic lobe, measurements in the parenchyma of the right hepatic lobe are recommended[24,25]. In addition, the statistical significance of this finding was marginal; thus, its immediate clinical significance is questionable.
Investigations of the effects of fasting time on measurements using VTTQ found an increase in SWV, especially in the first hour following food intake[13,28]. Following a time interval of three hours, however, no significant difference could be detected[28]. Similarly, data of the present study failed to detect any significant difference between the individual groups following a fasting period of at least three hours. Intra-individual variation in SWV of up to 0.3 m/s secondary to alimentary intake has been reported[13]. Measurements should therefore be acquired following a fasting period of at least three hours.
At a depth of 25 mm, the median SWV values were higher for measurements acquired using the 6C1 probe compared with those obtained using the 9L4 probe (P = 0.0067). The standard deviations for the two probes, however, were quite similar. Comparable findings were reported in another study[11]. In that study, however, the scatter of the linear and convex probes differed. Similarly, at a depth of 3.0 cm, lower values were measured with the linear probe than with the convex probe. A comparison of the 6C1 probe with the 9L4 probe at a depth of 15 mm yielded higher values for the linear probe (P = 0.0901). This may be explained by the proximity of the liver capsule. The scatter of the values was also greater (0.30 vs 0.20). Thus, the 6C1 is to be preferred for measurements in deeper liver regions.
Significantly higher SWV values were returned for measurements of the superficial hepatic parenchyma using the 9L4 probe than for measurements of deeper regions (P < 0.0001). In addition, significantly higher values were measured in the left hepatic lobe than on the right (P < 0.0001). Important factors include the liver capsule and the different physical characteristics of the ultrasound probes. In superficial regions there is also an increased risk of exerting pressure on the tissue with the probe tip. The anatomical position of the left hepatic lobe hinders visualization of the tissue and the measurement is affected by the pulsation of adjacent organs. An IQR < 30% is considered essential for the validity of the data[18,19]. For measurements at a depth of 15 mm in the right and left hepatic lobe, this yields values of 0.34 and 0.29. These values were higher than comparable values in deeper regions (0.22; 0.27). A minimum distance from the capsule of > 15 mm is recommended for measurements using the VTIQ technique.
In healthy volunteers, the 95%CI for the mean and median of the 6C1 and 9L4 probes stood at 1.23-1.29 m/s; 1.20-1.26 m/s and 1.17-1.23 m/s; 1.17 m/s-1.23 m/s, respectively. These data were below the established cut-off value of 1.34 m/s for diagnosis of high-grade liver fibrosis (F ≥ 2)[5]. In other studies, both higher and lower values were reported for mean ± SD, median, minimum, and maximum SWVs. It is therefore questionable whether SWV findings can be directly compared with other populations[7,20].
A limitation of the present study is the lack of laboratory data. Thus, subjects with unrecognized elevation of liver function tests could have been included in the study population. In addition, the validity of data was not secured using an SR of > 60% and an IQR of < 30%. However, unlike transient elastography, there are no corresponding guidelines from the manufacturer, to date. The reproducibility of the measurement method was also not investigated. The value of the VTTQ technique has already been shown in studies[6,7]. Corresponding investigations must now be performed for the VTIQ method.
Due to lower dispersion, measurements in the right hepatic lobe and deep regions are preferable. Gender differences must be considered. The BMI must be considered when assessing VTIQ. The choice of probe affects the SWV in deep measurements. Further studies must be performed to confirm the results.
Liver ultrasound elastography is one of the most recent acquisitions in the field of medical imaging. There are many approaches in liver elastography, based on different physical principles, but the goal remains the same: evaluation of liver elasticity, and thus assessment of liver fibrosis stage. Three main techniques are now subjects for numerous studies: transient elastography, shear-wave elastography, and acoustic radiation force impulse imaging. Factors that may affect shear wave measurements are currently important topics of scientific research.
Various factors affect the shear wave results and must be considered in applying the method.
Virtual touch tissue quantification (VTTQ) and virtual touch imaging quantification (VTIQ) are new elastographic techniques for estimating tissue stiffness. Both methods allow quantitative and non-invasive assessment of shear wave velocity. Shear wave speed quantification may be a diagnostic tool in the diagnosis of fibrotic liver changes. Therefore, standard values in healthy liver tissue must be generated.
Differences were found between measurements with convex and linear probes. Gender, BMI, and penetration influence the measurements.
VTIQ utilizes acoustic push pulses and tracking beams that are sequenced across a user-defined region of interest in order to generate an elastogram depicting the relative stiffness of tissue. VTTQ utilizes an acoustic push pulse to generate shear waves through a user-placed region of interest. When detection pulses interact with a passing shear wave, they reveal the wave’s location at a specific time, allowing calculation of the shear wave speed. This value is related to the stiffness of the tissue within the region of interest.
Many elastographic techniques have been developed to facilitate the non-invasive assessment of tissue properties. Just as the authors said: “To date, use of the new ARFI technique of VTIQ has only been described for normal mammary tissue”. This paper analyzed the VTTQ and VTIQ techniques and assessed the potential effects of various factors. The manuscript is well-written and guides the reader consequently throughout a profound framework of research data. The manuscript has a good revealed concept on the presented contexts.
P- Reviewer: Chen SJ S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Ma S
| 1. | Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D’Onofrio M, Drakonaki EE. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 765] [Article Influence: 63.8] [Reference Citation Analysis (1)] |
| 2. | Colombo S, Buonocore M, Del Poggio A, Jamoletti C, Elia S, Mattiello M, Zabbialini D, Del Poggio P. Head-to-head comparison of transient elastography (TE), real-time tissue elastography (RTE), and acoustic radiation force impulse (ARFI) imaging in the diagnosis of liver fibrosis. J Gastroenterol. 2012;47:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 468] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 4. | Takaki S, Kawakami Y, Miyaki D, Nakahara T, Naeshiro N, Murakami E, Tanaka M, Honda Y, Yokoyama S, Nagaoki Y. Non-invasive liver fibrosis score calculated by combination of virtual touch tissue quantification and serum liver functional tests in chronic hepatitis C patients. Hepatol Res. 2014;44:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212-e219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 363] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 6. | Bota S, Sporea I, Sirli R, Popescu A, Danila M, Costachescu D. Intra- and interoperator reproducibility of acoustic radiation force impulse (ARFI) elastography--preliminary results. Ultrasound Med Biol. 2012;38:1103-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | D'Onofrio M, Gallotti A, Mucelli RP. Tissue quantification with acoustic radiation force impulse imaging: Measurement repeatability and normal values in the healthy liver. AJR Am J Roentgenol. 2010;195:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Eiler J, Kleinholdermann U, Albers D, Dahms J, Hermann F, Behrens C, Luedemann M, Klingmueller V, Alzen GF. Standard value of ultrasound elastography using acoustic radiation force impulse imaging (ARFI) in healthy liver tissue of children and adolescents. Ultraschall Med. 2012;33:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Hanquinet S, Courvoisier D, Kanavaki A, Dhouib A, Anooshiravani M. Acoustic radiation force impulse imaging-normal values of liver stiffness in healthy children. Pediatr Radiol. 2013;43:539-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Lee MJ, Kim MJ, Han KH, Yoon CS. Age-related changes in liver, kidney, and spleen stiffness in healthy children measured with acoustic radiation force impulse imaging. Eur J Radiol. 2013;82:e290-e294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Chang S, Kim MJ, Kim J, Lee MJ. Variability of shear wave velocity using different frequencies in acoustic radiation force impulse (ARFI) elastography: a phantom and normal liver study. Ultraschall Med. 2013;34:260-265. [PubMed] |
| 12. | Goertz RS, Amann K, Heide R, Bernatik T, Neurath MF, Strobel D. An abdominal and thyroid status with Acoustic Radiation Force Impulse Elastometry--a feasibility study: Acoustic Radiation Force Impulse Elastometry of human organs. Eur J Radiol. 2011;80:e226-e230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Goertz RS, Egger C, Neurath MF, Strobel D. Impact of food intake, ultrasound transducer, breathing maneuvers and body position on acoustic radiation force impulse (ARFI) elastometry of the liver. Ultraschall Med. 2012;33:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Horster S, Mandel P, Zachoval R, Clevert DA. Comparing acoustic radiation force impulse imaging to transient elastography to assess liver stiffness in healthy volunteers with and without valsalva manoeuvre. Clin Hemorheol Microcirc. 2010;46:159-168. [PubMed] |
| 15. | Jaffer OS, Lung PF, Bosanac D, Patel VM, Ryan SM, Heneghan MA, Quaglia A, Sidhu PS. Acoustic radiation force impulse quantification: repeatability of measurements in selected liver segments and influence of age, body mass index and liver capsule-to-box distance. Br J Radiol. 2012;85:e858-e863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Kaminuma C, Tsushima Y, Matsumoto N, Kurabayashi T, Taketomi-Takahashi A, Endo K. Reliable measurement procedure of virtual touch tissue quantification with acoustic radiation force impulse imaging. J Ultrasound Med. 2011;30:745-751. [PubMed] |
| 17. | Gallotti A, D’Onofrio M, Pozzi Mucelli R. Acoustic Radiation Force Impulse (ARFI) technique in ultrasound with Virtual Touch tissue quantification of the upper abdomen. Radiol Med. 2010;115:889-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Madhok R, Tapasvi C, Prasad U, Gupta AK, Aggarwal A. Acoustic radiation force impulse imaging of the liver: measurement of the normal mean values of the shearing wave velocity in a healthy liver. J Clin Diagn Res. 2013;7:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Popescu A, Sporea I, Sirli R, Bota S, Focşa M, Dănilă M, Nicoliţă D, Martie A, Sendroiu M, Juchiş A. The mean values of liver stiffness assessed by Acoustic Radiation Force Impulse elastography in normal subjects. Med Ultrason. 2011;13:33-37. [PubMed] |
| 20. | Son CY, Kim SU, Han WK, Choi GH, Park H, Yang SC, Choi JS, Park JY, Kim do Y, Ahn SH. Normal liver elasticity values using acoustic radiation force impulse imaging: a prospective study in healthy living liver and kidney donors. J Gastroenterol Hepatol. 2012;27:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Golatta M, Schweitzer-Martin M, Harcos A, Schott S, Junkermann H, Rauch G, Sohn C, Heil J. Normal breast tissue stiffness measured by a new ultrasound technique: virtual touch tissue imaging quantification (VTIQ). Eur J Radiol. 2013;82:e676-e679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Treece G, Lindop J, Chen L, Housden J, Prager R, Gee A. Real-time quasi-static ultrasound elastography. Interface Focus. 2011;1:540-552. [PubMed] |
| 23. | Nightingale K. Acoustic Radiation Force Impulse (ARFI) Imaging: a Review. Curr Med Imaging Rev. 2011;7:328-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 24. | Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, Klauser AS, Sporea I, Calliada F, Cantisani V. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34:238-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 620] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 25. | Toshima T, Shirabe K, Takeishi K, Motomura T, Mano Y, Uchiyama H, Yoshizumi T, Soejima Y, Taketomi A, Maehara Y. New method for assessing liver fibrosis based on acoustic radiation force impulse: a special reference to the difference between right and left liver. J Gastroenterol. 2011;46:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008;48:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 331] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 27. | Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 1999;29:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 210] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Popescu A, Bota S, Sporea I, Sirli R, Danila M, Racean S, Suseanu D, Gradinaru O, Ivascu Siegfried C. The influence of food intake on liver stiffness values assessed by acoustic radiation force impulse elastography-preliminary results. Ultrasound Med Biol. 2013;39:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |