Copyright
©The Author(s) 2015.
World J Gastroenterol. Jul 7, 2015; 21(25): 7795-7804
Published online Jul 7, 2015. doi: 10.3748/wjg.v21.i25.7795
Published online Jul 7, 2015. doi: 10.3748/wjg.v21.i25.7795
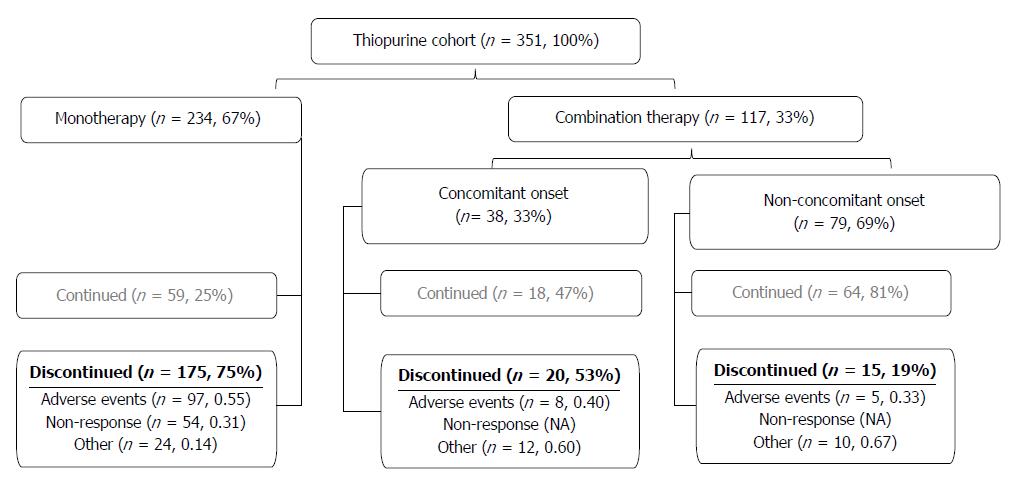
Figure 1 Patient disposition.
Disposition of the whole study cohort. Drug disposition is described in the materials and methods section. Thiopurines were discontinued due to adverse events, non-response or other non-clinical reasons.
- Citation: Moran GW, Dubeau MF, Kaplan GG, Yang H, Eksteen B, Ghosh S, Panaccione R. Clinical predictors of thiopurine-related adverse events in Crohn's disease. World J Gastroenterol 2015; 21(25): 7795-7804
- URL: https://www.wjgnet.com/1007-9327/full/v21/i25/7795.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i25.7795