Published online Jul 7, 2015. doi: 10.3748/wjg.v21.i25.7659
Peer-review started: January 14, 2015
First decision: March 10, 2015
Revised: April 4, 2015
Accepted: May 21, 2015
Article in press: May 21, 2015
Published online: July 7, 2015
Processing time: 175 Days and 3.4 Hours
Rectal adenocarcinoma is an important cause of cancer-related deaths worldwide, and key anatomic differences between the rectum and the colon have significant implications for management of rectal cancer. Many advances have been made in the diagnosis and management of rectal cancer. These include clinical staging with imaging studies such as endorectal ultrasound and pelvic magnetic resonance imaging, operative approaches such as transanal endoscopic microsurgery and laparoscopic and robotic assisted proctectomy, as well as refined neoadjuvant and adjuvant therapies. For stage II and III rectal cancers, combined chemoradiotherapy offers the lowest rates of local and distant relapse, and is delivered neoadjuvantly to improve tolerability and optimize surgical outcomes, particularly when sphincter-sparing surgery is an endpoint. The goal in rectal cancer treatment is to optimize disease-free and overall survival while minimizing the risk of local recurrence and toxicity from both radiation and systemic therapy. Optimal patient outcomes depend on multidisciplinary involvement for tailored therapy. The successful management of rectal cancer requires a multidisciplinary approach, with the involvement of enterostomal nurses, gastroenterologists, medical and radiation oncologists, radiologists, pathologists and surgeons. The identification of patients who are candidates for combined modality treatment is particularly useful to optimize outcomes. This article provides an overview of the diagnosis, staging and multimodal therapy of patients with rectal cancer for primary care providers.
Core tip: Colorectal cancer is the third most common malignant neoplasm and second most common cause of cancer-death in the United States. It is essential for primary care providers to become familiar with the modifications and updates in the diagnosis and treatment of this common malignancy. This review focuses on the advances made in the multidisciplinary approach to rectal cancer as well as minimally invasive surgical options as part of the management of rectal tumors.
- Citation: Gaertner WB, Kwaan MR, Madoff RD, Melton GB. Rectal cancer: An evidence-based update for primary care providers. World J Gastroenterol 2015; 21(25): 7659-7671
- URL: https://www.wjgnet.com/1007-9327/full/v21/i25/7659.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i25.7659
Colorectal cancer is the third most common noncutaneous malignancy in the United States and the second most frequent cause of cancer-related deaths. In 2015, an estimated 132700 cases of colorectal cancer will be diagnosed in the United States and will account for 49700 deaths[1]. Of these cancers, 30 percent will arise in the rectum. The diagnosis, staging and treatment regimens for rectal cancer differ significantly from those for colon cancer and have undergone recent advances that are important for primary care providers, gastroenterologists and general surgeons to be aware of.
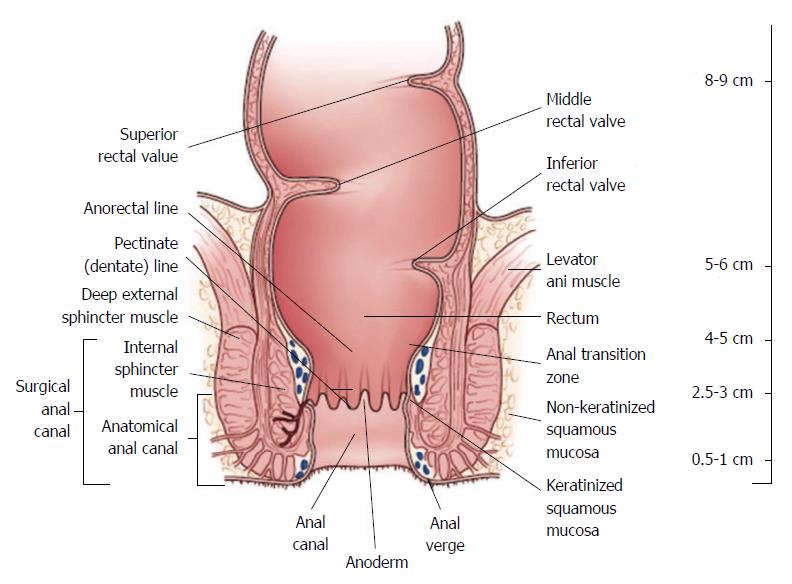
The work-up and management of rectal cancer requires detailed knowledge regarding its precise location. A National Cancer Institute consensus panel recommended that the rectum be defined as 12 cm or less from the anal verge using rigid proctoscopy (Figure 1)[2,3]. Anatomic considerations that distinguish rectal cancers from those that occur in the colon include the narrow and bony confines of the pelvis making surgical resection more difficult and the absence of serosa below the peritoneal reflection which facilitates deeper tumor growth in the perirectal fat, that may contribute to higher rates of locoregional failure[4].
The mainstay of treatment for patients with rectal cancer has been curative surgical resection. Significant improvements in local control and survival have been seen with the implementation of total mesorectal excision (TME) and the addition of neoadjuvant chemoradiotherapy (CRT)[5-9]. Increased use of colonoscopic screening has thought to contribute to disease detection at an earlier stage, which may contribute to improved outcomes as well.
The aim of this is review is to provide an evidence-based overview of the diagnosis, staging and multidisciplinary treatment of primary rectal cancer for primary care providers.
Many symptoms associated with colorectal cancer have been described, with the main ones being rectal bleeding, diarrhea, and constipation (commonly named “change in bowel habits”), as well as weight loss, abdominal pain, and anemia[10]. However, these symptoms are also common with benign conditions, therefore, clinicians must select patients at higher risk of colorectal cancer for further investigation. These risk factors include age ≥ 50 years, personal or family history of colorectal polyps and cancer, and history of inflammatory bowel disease. There is no reliable clinical information or test that has sufficient discrimination to provide the basis for referral decisions. Although primary care investigations such as fecal occult blood testing and estimation of hemoglobin are used to filter selected patients, symptomatic patients with risk factors for colorectal cancer should undergo a full colonoscopy.
Astin et al[11] performed a systematic review to identify the risk of colorectal cancer in patients reporting symptoms to primary care. Positive predictive values for rectal bleeding from 13 papers ranged from 2.2% to 16%, with a pooled estimate of 8.1% in those aged ≥ 50 years. The authors recommended further investigation of rectal bleeding or anemia in primary care patient’s ≥ 50 years.
Perhaps the most basic and informative test in patients with low rectal cancer is a digital examination (DRE). Important information can still be obtained from DRE, including the condition of the anal sphincters, distance from the anal verge with low-lying tumors, tumor fixation, and circumferential involvement. Nevertheless, DRE is not an adequate screening tool and even when rectal cancer is diagnosed, the associated findings do not correlate with the degree of tumor invasion.
Signs and symptoms associated with rectal cancer are non-specific but can guide primary care physicians in their referral decisions. Patient age, underlying inflammatory bowel disease and family history of colorectal cancer or polyps should influence this decision-making. Another important detail with patients that have significant family history is to consider referral to genetic counseling for appropriate risk assessment and timely notification of family members at risk.
When patients warrant endoscopic evaluation of the colon and rectum, a full colonoscopy is preferred to rule out the presence of synchronous polyps and cancers in the rest of the colon. Synchronous polyps or cancers may be present in 4% to 15% of patients[12]. Rigid proctoscopy will be performed in the surgeons’ office to accurately measure the distance form the anal verge and to characterize the lesion. Unlike colon cancer where the tattooing is performed liberally and sometimes even circumferentially for easy intraoperative visualization, rectal cancer should be tattooed right at the lesion with one single injection for accurate endoscopic visualization and for future visualization when neoadjuvant therapy is to be given.
Approximately 10% of polypectomy specimens harbor early colorectal carcinomas[13]. Endoscopic polypectomy alone is likely an adequate treatment for benign-appearing polyps ≤ 2 cm[14]. Patients with rectal polyps with malignant features (fixed, indurated, ulcerated), polyps > 2 cm, flat or serrated adenomas, and polyps that are unable to be completely excised endoscopically should be referred to a surgeon for excision. Patients with submucosal lesions, polyps close to the anal canal, and polypectomy specimens harboring invasive carcinoma or dysplasia should also be sent for surgical consultation. A more emergent situation is when a patient is diagnosed with dysplasia or invasive malignancy in a polypectomy specimen. These patients should be assessed immediately by a surgeon to visualize and tattoo the polypectomy site before healing occurs.
Rectal lesions that harbor invasive carcinoma, high-grade dysplasia or intramucosal carcinoma should be evaluated by a surgeon. Microscopic features associated with lymph node metastases, local recurrence and poor prognosis include poorly differentiated, mucinous and signet ring histology; lymphovascular and perineural invasion, and tumor budding. Tumor budding is defined as the presence of individual cells and small clusters of tumor cells at the invasive front of carcinomas. Ulcerated, mucinous cancers and lesions with evidence of perineural and lymphovascular invasion are associated with local recurrence rates as high as 25%[15,16]. Chemoradiotherapy regimens are less effective in tumors with undifferentiated histology and lymphovascular invasion[17].
The preoperative staging assessment of rectal carcinoma has significant implications in terms of treatment. Patients with rectal carcinomas that have not breached the muscularis propria layer of the rectal wall may be adequately treated by resection alone. On the other hand, patients who present with transmural invasion or those who have lymph node metastases benefit from neoadjuvant CRT followed by resection. Imaging studies utilized to evaluate patients with rectal tumors include computed tomography (CT), magnetic resonance imaging (MRI) and endorectal ultrasound (ERUS) (Table 1). MRI and ERUS are especially useful to detect tumor invasion outside the rectal wall and predict the relationship of the tumor with the circumferential margins[18]. With the more recent addition of diffusion-weighted imaging, MRI has also emerged as a reliable indicator for assessing early response following neoadjuvant CRT.
| CRM | T stage | N stage | EMVI | Peritoneum | |
| ERUS | NA | +++ | ++ | NA | NA |
| CT | + | ++ | - | + | + |
| MRI | +++ | +++ | ++++ | +++ | ++ |
| PET/CT | NA | NA | + | NA | NA |
ERUS is performed by a colorectal surgeon or gastroenterologist in an outpatient setting, requires minimal intestinal preparation, and results are highly operator dependant. MRI also requires minimal bowel preparation, does not suffer from operator variability, and patients are not exposed to radiation. When ordering an MRI to work-up a patient with rectal cancer, one must assure that a “rectal cancer protocol” is being performed as this imaging technique differs from regular pelvic MRI’s. MRI also has an increased cost when compared to ERUS.
Agreement between phase-array MRI and histopathology in predicting tumor stage has been established by a number of studies, including a prospective study by Brown et al[19] that showed a 94% agreement between MRI and pathologic assessment of T stage. The multicenter MERCURY study directly compared the extramural depth of invasion measured by MRI and histopathology in 295 of 311 patients[20]. The mean difference between MRI and histopathology was 0.046 mm, thereby showing MRI to be equivalent to a histopathology assessment of depth to within 0.5 mm in terms of predicting depth of extramural tumor spread.
In a meta-analysis including data from 90 publications, Bipat et al[21] found the sensitivity of ERUS and MRI for tumor invasion outside the rectal wall as high as 90% and 82%, respectively. However, the sensitivity for lymph node involvement was significantly lower at 67% and 66%, respectively. In a systematic review of 53 studies including 2915 patients[22], the accuracy of ERUS was 87% for T-stage and 74% for lymph node involvement. For MRI, the corresponding numbers were 84% and 82%. Recent data has shown that 3-D reconstruction increases the accuracy of ERUS in assessing the depth of rectal wall and submucosal invasion and may help in selecting patients for radical resection[23].
The addition of diffusion-weighted imaging [(DWI) a form of MRI based upon measuring the random Brownian motion of water molecules within a voxel of tissue] and dynamic contrast-enhanced imaging [(DCE) an MRI method that uses a contrast agent that enables the analysis of blood vessels], has recently shown to improve the accuracy in the local assessment of patients with rectal cancer, as well as the evaluation of treatment response after neoadjuvant therapy. Rao et al[24] showed that addition of DWI to T2-weighted imaging improved accuracy of rectal cancer detection. Ichikawa and colleagues studied DWI in 33 colorectal cancer patients (14 with rectal cancer) and reported 91% sensitivity and 100% specificity[25]. DWI has also been utilized for detection of metastatic lymph nodes in rectal cancer. Ono et al[26] reported 80% sensitivity, 77% specificity, and 78% accuracy in a series of 27 colorectal cancer patients (10 with rectal cancer). A recent study evaluating 129 patients showed 93% sensitivity, 81% specificity, and 87% accuracy in metastatic lymph node detection with combination of DWI and conventional MRI when compared with histopathologic examination after proctectomy with TME[27]. Diffusion-weighted imaging MRI has also been used to predict pathologic response after chemoradiation. Engin et al[28] showed that increase in apparent diffusion coefficient can predict therapy response. In many centers, DWI is now being used with T3 MRI protocols as an adjunctive to T2-weighted images. Dynamic contrast-enhanced MRI has been used in rectal cancer patients both for predicting response to therapy and for evaluation after neoadjuvant treatment. Kremser et al[29] applied dynamic T1 mapping as a predictor of post-chemoradiotherapy tumor-response. Gollub et al[30] showed that DCE MRI is reliable in predicting pathological complete response after chemotherapy. MRI with the use of a unique contrast agent, ultrasmall superparamagnetic ion oxide (USPIO) which undergoes phagocytosis by macrophages in normal lymph nodes, is a promising technique to help detect lymph node metastasis. T2 images are obtained 24 h after USPIO injection and reduced signal is accepted as normal whereas loss of signal indicates involvement of the lymph node. Koh et al[31] studied this technique in 25 patients with rectal cancer and reported improved accuracy with 65% sensitivity and 93% specificity. The use of USPIO has not been studied in a large comparative study nor has it been approved by the Food and Drug Administration for clinical use in the US.
CT scanning offers the opportunity in a single examination to stage rectal cancer both locally and distantly. It is readily available and relatively inexpensive and not prone to operator variability. When examining advanced rectal cancer, CT determines T stage with an accuracy of 79% to 94%; however, this falls to 52% to 74% when smaller tumors are evaluated18]. The assessment of lymph node involvement with CT has a very poor sensitivity, which ranges from 22% to 73%[32].
Overall, there is currently limited evidence in regards to the specificity and sensitivity of fluorodeoxyglucosepositron emission tomography (FDG-PET) in the initial staging of rectal cancer. PET/CT may be useful in detecting occult synchronous tumors or metastases at the time of initial presentation. However, this low-yield detection rate cannot justify the costs and radiation exposure for its routine use.
Pathologic stage represents the most important prognostic factor for patients who have rectal cancer. The tumor-node-metastasis (TNM) system, as defined by the American Joint Committee on Cancer (AJCC), is the most commonly used staging system and is based on depth of local invasion, extent of regional lymph node involvement, and presence of distant sites of disease (Table 2)[33]. As the AJCC stage increases from stage I to stage IV, 5-year overall survival declines from greater than 90% to less than 10%[34,35].
| Primary tumor (T) | |
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ: intraepithelial or invasion of lamina propria |
| T1 | Tumor invades submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades through the muscularis propria into the pericolorectal tissues |
| T4a | Tumor penetrates to the surface of the visceral peritoneum |
| T4b | Tumor directly invades or is adherent to other organs or structures |
| Regional lymph nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in 1-3 regional lymph nodes |
| N1a | Metastasis in one regional lymph node |
| N1b | Metastasis in 2-3 regional lymph nodes |
| N1c | Tumor deposit(s) in the subserosa, mesentery, or nonperitonealized pericolonic or perirectal tissues without without regional node metastasis |
| N2 | Metastasis in four or more regional lymph nodes |
| N2a | Metastasis in 4-6 regional lymph nodes |
| N2b | Metastasis in seven or more regional lymph nodes |
| Distant metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Metastasis confined to one organ or site (e.g., liver, lung, ovary, nonregional node) |
| M1b | Metastasis in more than one organ/site or the peritoneum |
The American Society of Clinical Oncology guidelines suggest a preoperative baseline carcinoembryonic antigen (CEA) level[36]. If increased preoperatively, the CEA level should return to normal range postoperatively. Serum levels of ≥ 5.0 ng/mL have an adverse impact on survival that is independent of tumor stage[36-39]. Elevated CEA levels that do not normalize following resection implies persistent disease and the need for further evaluation[40]. A CT scan of the chest, abdomen and pelvis to identify pulmonary and hepatic metastases should be performed. A chest CT is recommended because of the higher incidence of pulmonary metastases in rectal cancer patients compared to colon cancer patients[41].
The goals for treating rectal cancer have broadened to include securing local and distant oncologic control; minimizing treatment-related morbidity and mortality; performing restorative anastomosis to achieve near normal continence and defecation; preserving genitourinary functions; and promoting rapid recovery after resection with prompt return to normal activities.
Over the past two decades, neoadjuvant radiotherapy with or without sensitizing chemotherapy has been increasingly used together with surgical resection in the primary management of patients with rectal cancer. The rationale for radiotherapy is based on the finding that radiation inhibits cell proliferation, induces apoptotic cell death, and inhibits tumor growth[42]. The rationale for giving chemotherapy with radiotherapy is that it potentiates local radiotherapy sensitization and has the potential to induce tumor downsizing, possibly improving rates of sphincter preservation and increase rates of pathological complete response (pCR)[43].
In the US, neoadjuvant CRT is currently indicated for T3 and T4 rectal adenocarcinoma; and node positive tumors regardless of the T stage. There are several potential benefits to administering radiotherapy preoperatively, including decreased tumor seeding at operation, less acute toxicity, and increased likelihood of patient completion of the full treatment course[6,44,45]. One must have in mind that a major disadvantage is the potential for overtreatment of patients.
In the neoadjuvant setting, there are two possible approaches: short term radiation with 25Gy given in daily fractions of 5Gy and surgery the following week, or long term radiation treatment with chemotherapy in daily fractions of 1.8Gy five days per week, 50.4Gy in total, followed by surgery 6 to 8 wk later[46]. The latter treatment option, which has been defined as the standard of care for patients with locally advanced rectal cancer in the US, has the advantage of down staging of the tumor, particularly in advanced low rectal cancers[47]. The “long course” typically involves the administration of concurrent 5-FU-based chemotherapy[6,48,49]. The rationale for short-course radiotherapy is that the short time period for delivery of the dose may combat the effects of accelerated cellular repopulation, a phenomenon displayed by malignant cells exposed to radiotherapy. Short-course radiotherapy, which is widely used in Europe, does not result in apparent downsizing of tumors or downstaging in terms of nodal status, and has been associated with increased postoperative morbidity compared to “long course” radiotherapy[8]. Evidence from recent studies suggests that eliminating neoadjuvant radiotherapy may be feasible in selective patients with locally advanced rectal cancer. Specifically in patients with proximal rectal and chemosensitive tumors that may benefit from earlier and more intense systemic treatment. This regimen has the potential for reducing distant recurrence rates and avoiding the toxicity of pelvic radiotherapy, and is currently being studied in a randomized controlled trial[50].
Several randomized control trials have investigated the value of both radiotherapy and chemotherapy in the management of rectal cancer. In this section we will summarize these results.
Preoperative CRT is associated with a relative risk reduction in local recurrence of approximately 50% in patients with T3 and T4 rectal cancer compared with postoperative CRT. There is no significant difference in the overall rate of sphincter preservation or overall survival. In patients with T3 and T4 rectal cancer, the administration of chemotherapy in addition to preoperative long-course radiotherapy, regardless of the timing of administration (preoperative or postoperative), is associated with a relative risk reduction in local recurrence of approximately 50% compared with patients who received long-course preoperative radiotherapy alone. This significant difference in local recurrence has not translated into a significant difference in overall survival[6,8,45,48,49].
In patients with stage II and III rectal cancer, short-course radiotherapy with TME is associated with a relative risk reduction in local recurrence of 62% compared with patients who receive no neoadjuvant therapy. Patients who receive preoperative short-course radiotherapy have increased postoperative morbidity, mainly wound complications and bowel dysfunction[7,8,51-55].
In patients with T3 and T4 rectal cancer, there is no significant difference in the rate of sphincter preservation or in local recurrence between patients assigned to preoperative CRT compared to preoperative short-course radiotherapy[56].
The provision of chemotherapy and/or radiotherapy undoubtedly offers an oncological benefit in appropriately selected patients. The problem is that, in some instances, this benefit is at the cost of a 50% increase in some toxicity. Currently, there is no international consensus with regards to the indications for neoadjuvant CRT. International treatment guidelines are yet to be developed.
Several small, single-arm phase II trials have evaluated the outcomes of patients who receive neoadjuvant chemotherapy alone followed by TME and have reported down staging in 25%-58%, with 74%-84% disease-free survival and 85%-91% overall survival at between 4-5 years follow-up. Local recurrence rates at variable time intervals ranging from 48 to 75 mo have been reported in 0%-11.5% of patients. Postoperative complications have also been reported in up to 43% of patients in these small studies[57-61].
Currently, radical resection with TME remains the standard curative operation for rectal cancer. Patients with tumors located at the upper or mid rectum will frequently undergo an anterior or low anterior resection (LAR), whereas many patients with a distal tumor will require abdominoperineal resection (APR) of the rectum with a permanent colostomy (Table 3). Whether to perform local excision (LE), a restorative procedure with anterior resection (AR), or APR with permanent colostomy remains a complex assessment that must take into account oncologic and technical considerations, patient preference, functional outcome, and surgeon experience. The level of the lesion and its relationship to the anal sphincters and pelvic floor is a primary consideration from a technical and oncologic standpoint. Additional factors include initial staging, response to neoadjuvant therapy, tumor histology, and margin status. Patient factors, particularly a narrow pelvis and obesity, can add significant technical difficulty. Other factors include gender, age, anal sphincter function, and patient’s ability to manage a colostomy. Baseline bowel function, including incontinence, as well as sexual and urinary functions should be documented before any treatment modality.
| Anterior resection | Resection of rectum with an anastomosis above the pelvic peritoneal reflection |
| Low anterior resection | Resection of rectum with an anastomosis below the pelvic peritoneal reflection |
| TME | Total mesorectal resection. The adipose tissue at the posterior and lateral aspects of the rectum which contains the draining lymph nodes, is dissected down to the pelvic floor and resected |
| PME | Partial mesorectal excision. The mesorectum is divided 5 cm below the cancer as well as the distal rectum. PME is performed for cancers located in the upper rectum and rectosigmoid junction |
| TEM | Transanal endoscopic microsurgery. A specially designed proctoscope with an attached microscope permits local resection of premalignant lesions and selected cases of early rectal cancer up to 20 cm from the anal verge |
| TAE | Transanal excision. Lesions in the lower third of rectum can be resected transanally |
| APR | Abdominoperineal resection. Low rectal cancers that cannot be resected with a sphincter-saving procedure are resected with perianal tissue and the anal canal en bloc with the whole rectum and mesorectum |
| Adjuvant | Additional treatment (chemotherapy, radiation therapy or chemoradiation) given after surgical resection |
| Neoadjuvant | Preoperative treatment |
| CRT | Chemoradiotherapy. Chemotherapy drugs typically involve 5-fluorouracil, leucovorin and oxaliplatin. These are given in order to increase cancer cells sensitivity to the radiation. CRT is frequently offered to patients preoperatively (neoadjuvant) in order to reduce local recurrence but has not shown to improve overall survival |
| Intersphincteric resection | The internal anal sphincter muscle is resected in continuity with the lower rectum preserving the external anal sphincter in order to preserve anal function and avoid colostomy in cases of ultralow rectal cancer |
| CRM | Circumferential resection margin is the distance in mm from the mesorectal fascia (the resection plane) to the nearest tumor growth |
| DRM | Distal resection margin |
Early rectal cancer is relatively uncommon in Western populations. The incidence of malignant colorectal polyps as a proportion of all adenomas removed varies between 2.6% and 9.7%[62], with 3% to 8.6% of all resected colorectal adenocarcinomas staged as T1[63-66]. The role of LE for treatment of rectal cancer is highly controversial. While radical resection with TME continues to be the standard operation for most patients with rectal cancer, LE is an acceptable alternative with significantly less morbidity. Most surgeons restrict their curative intent use to selected patients with T1 disease (Table 4) or to those patients unfit for radical resection.
| Favorable/low risk | Unfavorable/high risk |
| Well differentiated (G1-G2) | Poorly differentiated (G3) |
| SM 1 | SM 2-3 |
| Size < 3 cm | Size > 3 cm |
| < 40% wall circumferences | > 40% wall circumferences |
| No lymphovascular invasion | Lymphovascular invasion |
| No tumor budding | Tumor budding |
| No perineural invasion | Perineural invasion |
| No lymphocitic infiltration | Lymphocitic infiltration |
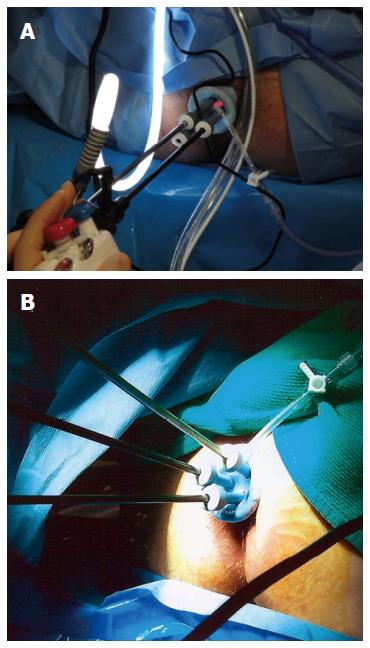
Surgeons continue to evolve techniques for LE. The most common technique for LE is transanal excision (TAE), which involves the excision of the rectal tumor with the assistance of an operating anoscope. This technique is exclusive for low-lying tumors and suffers from poor visualization. Transanal endoscopic microsurgery (TEM) is a modification of LE that combines the excellent visualization offered by a binocular stereoscope that is incorporated into an operating proctoscope, which permits an optimum view during the procedure thus enhancing the surgeon’s ability to accurately perform full thickness excisions and to repair the rectal wall defect. TEM allows for improved endoanal access to the mid and upper rectum thus increasing the utility of LE. Disadvantages of TEM include costly equipment and slightly longer operating times. Atallah et al[67] recently reported their experience with using a single-incision laparoscopic surgery port for access to the rectum, replacing the conventional operative proctoscope, and using ordinary laparoscopic instruments (Figure 2). This approach is widely known as transanal minimally invasive surgery (TAMIS) and has been reported to be a safe and feasible alternative to TEM, providing its benefits at a fraction of the cost[68,69].
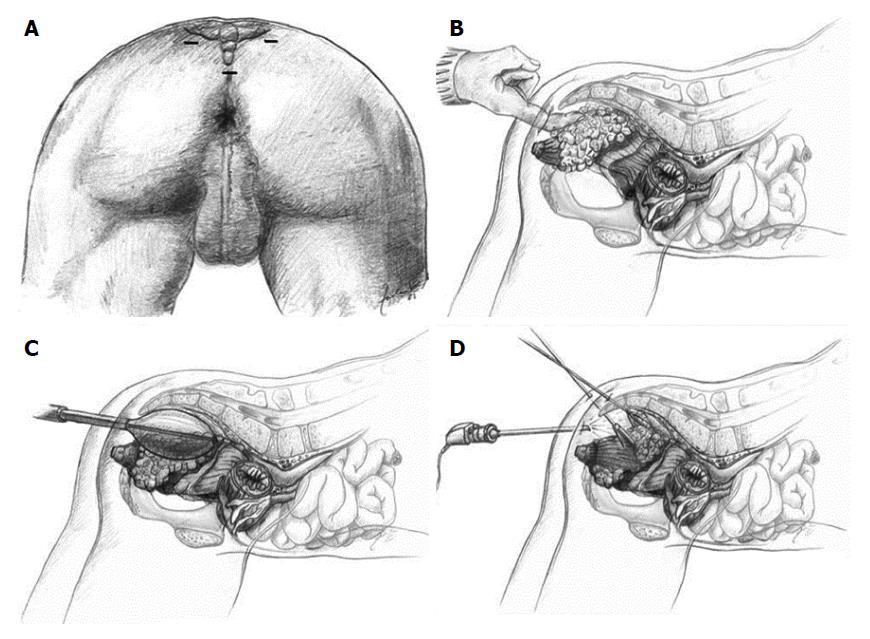
One significant disadvantage of LE, including TEM, is the lack of information regarding the lymph node status of the mesorectum. Endoscopic posterior mesorectal resection (EPMR) is a recently described technique[70] that includes TAE or TEM of selected, favorable T1 rectal cancers followed by a minimally invasive transperineal resection of the posterior part of the mesorectum including all relevant lymphatic tissue (Figure 3). Its proponents claim this technique provides complete tumor staging with minimal morbidity after LE of T1 rectal cancers[71].
Despite its decreased morbidity and mortality, LE has been associated with significantly higher local recurrence rates (12.5% vs 6.9% for T1 cancers and 22.1% vs 15.1% for T2 cancers)[72]. Compared to TAE, TEM and TAMIS offer a higher likelihood of achieving clear resection margins, lower recurrence rates and the ability to successfully excise more proximal tumors. Local recurrence after TEM and TAMIS has been reported mainly in single institution reviews which makes comparisons difficult. Recurrence rates range from 0% to 13% for patients with T1 tumors and from 0% to 80% for patients with T2 tumors[73-78].
Significant disease progression can occur after any type of LE despite intense surveillance[79,80], which may preclude curative salvage. The role of CRT and LE techniques in the treatment of rectal cancer is still under study.
The determining factor in performing a sphincter-preserving operation is the ability to obtain adequate distal margin. For mid to low tumors or patients with difficult anatomy, the decision of whether to perform a sphincter preserving operation or not is generally only possible in the operating room when the rectum is completely mobilized. When performed for curative intent, both AR and APR involve TME to achieve adequate circumferential margin clearance. TME involves excision of the mesorectum following the anatomic planes of the pelvis. Dissection is performed sharply with the identification and preservation of the autonomic nervous system of the pelvis. TME has been repeatedly associated with a reduction in the local recurrence rate from 30%-40% to 5%-15% with the suggestion that surgical technique is a key factor[81,82]. TME has not shown significant differences in 30-d mortality, anastomotic leakage or overall operative morbidity when compared to pre TME-era controls with or without neoadjuvant therapy[83-85].
Large comparative studies and multiple prospective randomized control trials have reported equivalence in short and long-term outcomes between open and laparoscopic resections for colon cancer[86-91] but laparoscopic AR with TME has not been well studied and whether it compromises long-term oncologic outcomes has not been refuted by the available literature. Laparoscopic rectal dissection is technically more demanding and may result in difficulties assessing and achieving negative surgical margins but does provide a clear and magnified view of the pelvis that helps with the sharp dissection for TME and assist in identification of vital pelvic structures including the ureters and autonomic nerves. Current data suggests that laparoscopic rectal cancer resection may benefit patients with reduced blood loss, earlier return of bowel function, and shorter hospital length of stay[92,93]. There are two large, multicenter randomized controlled trials that are currently being conducted: the COLOR II trial in Europe and the ACOSOG-Z6051 trial in the US[94]. Both of these studies are comparing the laparoscopic and open approach for treatment of resectable rectal cancer.
In recent years, an increasing number of reports have been published on robotic colorectal surgery. Most of the interest has been in robotic TME for rectal cancer. Robotic-assisted laparoscopy can ease some of the limitations of conventional laparoscopy with improved visualization and maximal maneuverability provided by 360-degree reticulating arms. In colorectal surgery, robotic techniques are associated with longer operative times and higher costs compared with laparoscopic surgery[95]. Although operative morbidity and short-term oncologic outcomes are comparable to the laparoscopic approach, long-term outcomes remain unknown. Large prospective randomized clinical trials such as the international ROLARR trial are required to establish the benefits of robotic rectal surgery. The role of robotics in colorectal surgery is still largely undefined.
The long term follow up of the European Organization for Research and Treatment of Cancer trial 22921 that compared adjuvant 5-FU-based chemotherapy to no adjuvant treatment in patients with resectable T3-4 rectal cancer, reported no beneficial effects of adjuvant chemotherapy if the cancer did not respond to preoperative radiation or CRT[96]. The role of intraoperative and postoperative radiation has not been well studied and is limited to inadvertent intraoperative tumor perforations or involved resection margins if irradiation treatment was not given preoperatively[97-100].
Many studies have shown that neoadjuvant CRT is associated with significant pathological downstaging of rectal cancers, with up to 20% of patients having pCR[101-104]. Despite an apparent complete luminal and mural tumor response, up to 17% of tumors with histologically confirmed pCR harbor disease in the mesorectal lymph nodes[105]. Similarly, approximately 8% of patients with an apparent incomplete clinical response have pCR[106]. The challenge remains to identify those patients with a clinical complete response who have a true pCR.
Habr-Gama and colleagues from Sao Paulo, Brazil have pioneered the “watch and wait” approach where they enroll patients with pCR into a strict surveillance protocol without subsequent operative treatment. In their most updated experience including 67 patients with pCR, overall survival and disease-free survival rates at 5 years were 96% and 72%, respectively[107]. After a mean follow-up of 65 mo, recurrences were observed in 15 patients (21%). All endorectal recurrences were amenable to salvage therapy.
Although the results of Dr. Habr-Gama’s nonoperative group appear promising, these data should be received with caution because others have shown that greater than 80% of complete clinical responders will recur locally within the first 10 mo of observation[108,109]. This implies that the nonoperative approach may only be appropriate for a subset of patients who have a durable pCR after neoadjuvant CRT. It is likely that combined radiological, biochemical and molecular biological markers will be required to accurately predict pCR as well as nodal status.
Improved imaging techniques for staging, precise histopathologic assessments and feedback, and multidisciplinary treatment strategies have led to a greater understanding of the natural history of rectal cancer and improved outcomes. With accurate preoperative imaging techniques for staging, such as ERUS and MRI, patient selection for neoadjuvant CRT is constantly improving. Neoadjuvant CRT has been well studied and has been associated with significantly decreased local recurrence but no significant improvement in overall survival. A “watch and wait” approach in selected patients with pCR after neoadjuvant CRT has been postulated but long-term results and expanded experiences are pending. Improved operative results with TME and the ongoing experience with laparoscopic and robotic-assisted techniques have also led to improved outcomes with faster recovery. LE with TAE, TEM or TAMIS should be performed selectively on T1 tumors with favorable clinical and histopathologic features. Management of rectal cancer can be complex and is optimized when approached in a coordinated manner by an experienced multidisciplinary cancer treatment team.
P- Reviewer: Cai SJ S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9957] [Article Influence: 995.7] [Reference Citation Analysis (0)] |
| 2. | Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, Miedema B, Ota D, Sargent D. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 928] [Cited by in RCA: 947] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 3. | Apgar BS, Brotzman GL, Spitzer M, editors . Colposcopy principles and practice, 2nd ed. Saunders: Philadelphia 2008; . |
| 4. | Glynne-Jones R, Mathur P, Elton C, Train ML. The multidisciplinary management of gastrointestinal cancer. Multimodal treatment of rectal cancer. Best Pract Res Clin Gastroenterol. 2007;21:1049-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 1913] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 6. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4457] [Article Influence: 212.2] [Reference Citation Analysis (1)] |
| 7. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3104] [Cited by in RCA: 3116] [Article Influence: 129.8] [Reference Citation Analysis (0)] |
| 8. | Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1110] [Article Influence: 69.4] [Reference Citation Analysis (2)] |
| 9. | Scott N, Jackson P, al-Jaberi T, Dixon MF, Quirke P, Finan PJ. Total mesorectal excision and local recurrence: a study of tumour spread in the mesorectum distal to rectal cancer. Br J Surg. 1995;82:1031-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 200] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 10. | Hamilton W, Sharp D. Diagnosis of colorectal cancer in primary care: the evidence base for guidelines. Fam Pract. 2004;21:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Astin M, Griffin T, Neal RD, Rose P, Hamilton W. The diagnostic value of symptoms for colorectal cancer in primary care: a systematic review. Br J Gen Pract. 2011;61:e231-e243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Takeuchi H, Toda T, Nagasaki S, Kawano T, Minamisono Y, Maehara Y, Sugimachi K. Synchronous multiple colorectal adenocarcinomas. J Surg Oncol. 1997;64:304-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Muto T, Sawada T, Sugihara K. Treatment of carcinoma in adenomas. World J Surg. 1991;15:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Greene FL. Colonoscopic polypectomy: indication, technique, and therapeutic implications. Semin Surg Oncol. 1995;11:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Sengupta S, Tjandra JJ. Local excision of rectal cancer: what is the evidence? Dis Colon Rectum. 2001;44:1345-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Hermanek P, Gall FP. Significance of local control of colorectal cancer. Fortschr Med. 1985;103:1041-1046. [PubMed] |
| 17. | Chakravarti A, Compton CC, Shellito PC, Wood WC, Landry J, Machuta SR, Kaufman D, Ancukiewicz M, Willett CG. Long-term follow-up of patients with rectal cancer managed by local excision with and without adjuvant irradiation. Ann Surg. 1999;230:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 166] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Muthusamy VR, Chang KJ. Optimal methods for staging rectal cancer. Clin Cancer Res. 2007;13:6877s-6884s. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 519] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 20. | MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 329] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 21. | Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 721] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 22. | Kwok H, Bissett IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis. 2000;15:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 222] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Santoro GA, D’Elia A, Battistella G, Di Falco G. The use of a dedicated rectosigmoidoscope for ultrasound staging of tumours of the upper and middle third of the rectum. Colorectal Dis. 2007;9:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Rao SX, Zeng MS, Chen CZ, Li RC, Zhang SJ, Xu JM, Hou YY. The value of diffusion-weighted imaging in combination with T2-weighted imaging for rectal cancer detection. Eur J Radiol. 2008;65:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Ichikawa T, Erturk SM, Motosugi U, Sou H, Iino H, Araki T, Fujii H. High-B-value diffusion-weighted MRI in colorectal cancer. AJR Am J Roentgenol. 2006;187:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 26. | Ono K, Ochiai R, Yoshida T, Kitagawa M, Omagari J, Kobayashi H, Yamashita Y. Comparison of diffusion-weighted MRI and 2-[fluorine-18]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) for detecting primary colorectal cancer and regional lymph node metastases. J Magn Reson Imaging. 2009;29:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Mizukami Y, Ueda S, Mizumoto A, Sasada T, Okumura R, Kohno S, Takabayashi A. Diffusion-weighted magnetic resonance imaging for detecting lymph node metastasis of rectal cancer. World J Surg. 2011;35:895-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Engin G, Sharifov R, Güral Z, Sağam EK, Sağlam S, Balik E, Asoğu O, Yamaner S, Güllüoğu M, Kapran Y. Can diffusion-weighted MRI determine complete responders after neoadjuvant chemoradiation for locally advanced rectal cancer? Diagn Interv Radiol. 2012;18:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 29. | Kremser C, Trieb T, Rudisch A, Judmaier W, de Vries A. Dynamic T(1) mapping predicts outcome of chemoradiation therapy in primary rectal carcinoma: sequence implementation and data analysis. J Magn Reson Imaging. 2007;26:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Gollub MJ, Gultekin DH, Akin O, Do RK, Fuqua JL, Gonen M, Kuk D, Weiser M, Saltz L, Schrag D. Dynamic contrast enhanced-MRI for the detection of pathological complete response to neoadjuvant chemotherapy for locally advanced rectal cancer. Eur Radiol. 2012;22:821-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Koh DM, George C, Temple L, Collins DJ, Toomey P, Raja A, Bett N, Farhat S, Husband JE, Brown G. Diagnostic accuracy of nodal enhancement pattern of rectal cancer at MRI enhanced with ultrasmall superparamagnetic iron oxide: findings in pathologically matched mesorectal lymph nodes. AJR Am J Roentgenol. 2010;194:W505-W513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Thompson WM, Halvorsen RA, Foster WL, Roberts L, Gibbons R. Preoperative and postoperative CT staging of rectosigmoid carcinoma. AJR Am J Roentgenol. 1986;146:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 100] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Greene F, Page D, Fleming I, editors . AJCC cancer staging handbook. 7th ed. New York: Springer 2010; . |
| 34. | Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 859] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 35. | O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1162] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 36. | Harrison LE, Guillem JG, Paty P, Cohen AM. Preoperative carcinoembryonic antigen predicts outcomes in node-negative colon cancer patients: a multivariate analysis of 572 patients. J Am Coll Surg. 1997;185:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Wiggers T, Arends JW, Volovics A. Regression analysis of prognostic factors in colorectal cancer after curative resections. Dis Colon Rectum. 1988;31:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 115] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Wolmark N, Fisher B, Wieand HS, Henry RS, Lerner H, Legault-Poisson S, Deckers PJ, Dimitrov N, Gordon PH, Jochimsen P. The prognostic significance of preoperative carcinoembryonic antigen levels in colorectal cancer. Results from NSABP (National Surgical Adjuvant Breast and Bowel Project) clinical trials. Ann Surg. 1984;199:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Slentz K, Senagore A, Hibbert J, Mazier WP, Talbott TM. Can preoperative and postoperative CEA predict survival after colon cancer resection? Am Surg. 1994;60:528-531; discussion 531-532. [PubMed] |
| 40. | Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest. 2005;23:338-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 294] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 41. | Penna C, Nordlinger B. Colorectal metastasis (liver and lung). Surg Clin North Am. 2002;82:1075-1090, x-xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Hendry JH, West CM. Apoptosis and mitotic cell death: their relative contributions to normal-tissue and tumour radiation response. Int J Radiat Biol. 1997;71:709-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 111] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Rectal Cancer. Fort Washington, PA: National Comprehensive Cancer Network 2009; . |
| 44. | Valentini V, Beets-Tan R, Borras JM, Krivokapić Z, Leer JW, Påhlman L, Rödel C, Schmoll HJ, Scott N, Velde CV. Evidence and research in rectal cancer. Radiother Oncol. 2008;87:449-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Roh MS, Colangelo LH, O’Connell MJ, Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L, Ursiny CS, Petrelli NJ. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124-5130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 700] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 46. | Arnoletti JP, Bland KI. Neoadjuvant and adjuvant therapy for rectal cancer. Surg Oncol Clin N Am. 2006;15:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Habr-Gama A, Perez RO, Kiss DR, Rawet V, Scanavini A, Santinho PM, Nadalin W. Preoperative chemoradiation therapy for low rectal cancer. Impact on downstaging and sphincter-saving operations. Hepatogastroenterology. 2004;51:1703-1707. [PubMed] |
| 48. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2040] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 49. | Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620-4625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1273] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 50. | Chemotherapy Alone or Chemotherapy Plus Radiation Therapy in Treating Patients With Locally Advanced Rectal Cancer Undergoing Surgery. Cited 2014 9/14/2014. Available from: http://clinicaltrials.gov/show/NCT01515787. |
| 51. | Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997;336:980-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1816] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 52. | Initial report from a Swedish multicentre study examining the role of preoperative irradiation in the treatment of patients with resectable rectal carcinoma. Swedish Rectal Cancer Trial. Br J Surg. 1993;80:1333-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 143] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Local recurrence rate in a randomised multicentre trial of preoperative radiotherapy compared with operation alone in resectable rectal carcinoma. Swedish Rectal Cancer Trial. Eur J Surg. 1996;162:397-402. [PubMed] |
| 54. | Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644-5650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 586] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 55. | Peeters KC, van de Velde CJ, Leer JW, Martijn H, Junggeburt JM, Kranenbarg EK, Steup WH, Wiggers T, Rutten HJ, Marijnen CA. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J Clin Oncol. 2005;23:6199-6206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 666] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 56. | Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 870] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 57. | Ishii Y, Hasegawa H, Endo T, Okabayashi K, Ochiai H, Moritani K, Watanabe M, Kitagawa Y. Medium-term results of neoadjuvant systemic chemotherapy using irinotecan, 5-fluorouracil, and leucovorin in patients with locally advanced rectal cancer. Eur J Surg Oncol. 2010;36:1061-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Uehara K, Hiramatsu K, Maeda A, Sakamoto E, Inoue M, Kobayashi S, Tojima Y, Yoshioka Y, Nakayama G, Yatsuya H. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: N-SOG 03 Phase II trial. Jpn J Clin Oncol. 2013;43:964-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 59. | Koike J, Hiramatsu K, Maeda A, Sakamoto E, Inoue M, Kobayashi S, Tojima Y, Yoshioka Y, Nakayama G, Yatsuya H. Neoadjuvant mFOLFOX6 for stage II/III rectal cancer patients with a T3/T4 tumor. J Clin Oncol. 2014;32:abstr 3554. |
| 60. | Cercek A, Weiser MR, Goodman KA, Reidy DL, Wong WD, Guillem JG, Temple LK, Schrag D, Paty P, Saltz L. Complete pathologic response in the primary of rectal or colon cancer treated with FOLFOX without radiation. J Clin Oncol. 2010;28:abstr 3649. |
| 61. | Schrag D, Weiser MR, Goodman KA, Gonen M, Hollywood E, Cercek A, Reidy-Lagunes DL, Gollub MJ, Shia J, Guillem JG. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 305] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 62. | Bernstein TE, Endreseth BH, Romundstad P, Wibe A. Circumferential resection margin as a prognostic factor in rectal cancer. Br J Surg. 2009;96:1348-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 63. | Haboubi , Scott . Clinicopathological management of the patient with a malignant colorectal adenoma. Colorectal Dis. 2000;2:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Morson BC, Bussey HJ. Predisposing causes of intestinal cancer. Curr Probl Surg. 1970;1-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002;45:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 466] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 66. | Huddy SP, Husband EM, Cook MG, Gibbs NM, Marks CG, Heald RJ. Lymph node metastases in early rectal cancer. Br J Surg. 1993;80:1457-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg Endosc. 2010;24:2200-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 389] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 68. | Martin-Perez B, Andrade-Ribeiro GD, Hunter L, Atallah S. A systematic review of transanal minimally invasive surgery (TAMIS) from 2010 to 2013. Tech Coloproctol. 2014;18:775-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 69. | Rimonda R, Arezzo A, Arolfo S, Salvai A, Morino M. TransAnal Minimally Invasive Surgery (TAMIS) with SILS™ port versus Transanal Endoscopic Microsurgery (TEM): a comparative experimental study. Surg Endosc. 2013;27:3762-3768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 70. | Zerz A, Müller-Stich BP, Beck J, Linke GR, Tarantino I, Lange J. Endoscopic posterior mesorectal resection after transanal local excision of T1 carcinomas of the lower third of the rectum. Dis Colon Rectum. 2006;49:919-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 71. | Tarantino I, Hetzer FH, Warschkow R, Zünd M, Stein HJ, Zerz A. Local excision and endoscopic posterior mesorectal resection versus low anterior resection in T1 rectal cancer. Br J Surg. 2008;95:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | You YN, Baxter NN, Stewart A, Nelson H. Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: a nationwide cohort study from the National Cancer Database. Ann Surg. 2007;245:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 73. | Winde G, Nottberg H, Keller R, Schmid KW, Bünte H. Surgical cure for early rectal carcinomas (T1). Transanal endoscopic microsurgery vs. anterior resection. Dis Colon Rectum. 1996;39:969-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 271] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 74. | Lezoche G, Baldarelli M, Guerrieri M, Paganini AM, De Sanctis A, Bartolacci S, Lezoche E. A prospective randomized study with a 5-year minimum follow-up evaluation of transanal endoscopic microsurgery versus laparoscopic total mesorectal excision after neoadjuvant therapy. Surg Endosc. 2008;22:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 196] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 75. | Bach SP, Hill J, Monson JR, Simson JN, Lane L, Merrie A, Warren B, Mortensen NJ. A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg. 2009;96:280-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 76. | Ganai S, Kanumuri P, Rao RS, Alexander AI. Local recurrence after transanal endoscopic microsurgery for rectal polyps and early cancers. Ann Surg Oncol. 2006;13:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 77. | Stipa F, Burza A, Lucandri G, Ferri M, Pigazzi A, Ziparo V, Casula G, Stipa S. Outcomes for early rectal cancer managed with transanal endoscopic microsurgery: a 5-year follow-up study. Surg Endosc. 2006;20:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 78. | Tsai BM, Finne CO, Nordenstam JF, Christoforidis D, Madoff RD, Mellgren A. Transanal endoscopic microsurgery resection of rectal tumors: outcomes and recommendations. Dis Colon Rectum. 2010;53:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 79. | Borschitz T, Wachtlin D, Möhler M, Schmidberger H, Junginger T. Neoadjuvant chemoradiation and local excision for T2-3 rectal cancer. Ann Surg Oncol. 2008;15:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 80. | Lee WY, Lee WS, Yun SH, Shin SH, Chun HK. Decision for salvage treatment after transanal endoscopic microsurgery. Surg Endosc. 2007;21:975-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Heald RJ. Total mesorectal excision. The new European gold standard. G Chir. 1998;19:253-255. [PubMed] |
| 82. | Enker WE. Total mesorectal excision--the new golden standard of surgery for rectal cancer. Ann Med. 1997;29:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 152] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 83. | Martling A, Holm T, Johansson H, Rutqvist LE, Cedermark B. The Stockholm II trial on preoperative radiotherapy in rectal carcinoma: long-term follow-up of a population-based study. Cancer. 2001;92:896-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 84. | Cedermark B, Johansson H, Rutqvist LE, Wilking N. The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma. A prospective randomized trial. Stockholm Colorectal Cancer Study Group. Cancer. 1995;75:2269-2275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 85. | Randomized study on preoperative radiotherapy in rectal carcinoma. Stockholm Colorectal Cancer Study Group. Ann Surg Oncol. 1996;3:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 170] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 86. | Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2295] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 87. | Lacy AM, García-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, Visa J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1901] [Cited by in RCA: 1816] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 88. | Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1679] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 89. | Law WL, Lee YM, Choi HK, Seto CL, Ho JW. Impact of laparoscopic resection for colorectal cancer on operative outcomes and survival. Ann Surg. 2007;245:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 90. | Milsom JW, Böhm B, Hammerhofer KA, Fazio V, Steiger E, Elson P. A prospective, randomized trial comparing laparoscopic versus conventional techniques in colorectal cancer surgery: a preliminary report. J Am Coll Surg. 1998;187:46-54; discussion 54-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 436] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 91. | Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061-3068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1111] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 92. | Staudacher C, Di Palo S, Tamburini A, Vignali A, Orsenigo E. Total mesorectal excision (TME) with laparoscopic approach: 226 consecutive cases. Surg Oncol. 2007;16 Suppl 1:S113-S116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 93. | Pugliese R, Di Lernia S, Sansonna F, Maggioni D, Ferrari GC, Magistro C, Costanzi A, De Carli S, Artale S, Pugliese F. Laparoscopic resection for rectal adenocarcinoma. Eur J Surg Oncol. 2009;35:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Buunen M, Bonjer HJ, Hop WC, Haglind E, Kurlberg G, Rosenberg J, Lacy AM, Cuesta MA, D’Hoore A, Fürst A. COLOR II. A randomized clinical trial comparing laparoscopic and open surgery for rectal cancer. Dan Med Bull. 2009;56:89-91. [PubMed] |
| 95. | Baek SK, Carmichael JC, Pigazzi A. Robotic surgery: colon and rectum. Cancer J. 2013;19:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 96. | Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, Radosevic-Jelic L, Piérart M, Calais G. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. 2007;25:4379-4386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 331] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 97. | Williams CP, Reynolds HL, Delaney CP, Champagne B, Obias V, Joh YG, Merlino J, Kinsella TJ. Clinical results of intraoperative radiation therapy for patients with locally recurrent and advanced tumors having colorectal involvement. Am J Surg. 2008;195:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 98. | Lee JH, Jo IY, Lee JH, Yoon SC, Kim YS, Choi BO, Kim JG, Oh ST, Lee MA, Jang HS. The role of postoperative pelvic radiation in stage IV rectal cancer after resection of primary tumor. Radiat Oncol J. 2012;30:205-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 99. | Chang CY, Kim HC, Park YS, Park JO, Choi DH, Park HC, Cho YB, Yun SH, Lee WY, Chun HK. The effect of postoperative pelvic irradiation after complete resection of metastatic rectal cancer. J Surg Oncol. 2012;105:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 100. | Yeung JM, Ngan S, Lynch C, Heriot AG. Intraoperative radiotherapy and colorectal cancer. Minerva Chir. 2010;65:161-171. [PubMed] |
| 101. | Chan AK, Wong A, Jenken D, Heine J, Buie D, Johnson D. Posttreatment TNM staging is a prognostic indicator of survival and recurrence in tethered or fixed rectal carcinoma after preoperative chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:665-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 102. | de Campos-Lobato LF, Stocchi L, da Luz Moreira A, Geisler D, Dietz DW, Lavery IC, Fazio VW, Kalady MF. Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann Surg Oncol. 2011;18:1590-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 103. | Garcia-Aguilar J, Smith DD, Avila K, Bergsland EK, Chu P, Krieg RM. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg. 2011;254:97-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 254] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 104. | Smith KD, Tan D, Das P, Chang GJ, Kattepogu K, Feig BW, Skibber JM, Rodriguez-Bigas MA. Clinical significance of acellular mucin in rectal adenocarcinoma patients with a pathologic complete response to preoperative chemoradiation. Ann Surg. 2010;251:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 105. | Bedrosian I, Rodriguez-Bigas MA, Feig B, Hunt KK, Ellis L, Curley SA, Vauthey JN, Delclos M, Crane C, Janjan N. Predicting the node-negative mesorectum after preoperative chemoradiation for locally advanced rectal carcinoma. J Gastrointest Surg. 2004;8:56-62; discussion 62-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 106. | Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Silva e Sousa AH, Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711-717; discussion 717-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1358] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 107. | Habr-Gama A, Perez RO, São Julião GP, Proscurshim I, Gama-Rodrigues J. Nonoperative approaches to rectal cancer: a critical evaluation. Semin Radiat Oncol. 2011;21:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 108. | Rossi BM, Nakagawa WT, Novaes PE, Filho WD, Lopes A. Radiation and chemotherapy instead of surgery for low infiltrative rectal adenocarcinoma: a prospective trial. Ann Surg Oncol. 1998;5:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 109. | Nakagawa WT, Rossi BM, de O Ferreira F, Ferrigno R, David Filho WJ, Nishimoto IN, Vieira RA, Lopes A. Chemoradiation instead of surgery to treat mid and low rectal tumors: is it safe? Ann Surg Oncol. 2002;9:568-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |