Published online Jun 14, 2015. doi: 10.3748/wjg.v21.i22.7022
Peer-review started: October 19, 2014
First decision: December 2, 2014
Revised: December 12, 2014
Accepted: February 12, 2015
Article in press: February 13, 2015
Published online: June 14, 2015
AIM: To review and assess the evidence related to cetuximab treatment in metastatic colorectal cancer (mCRC) with regard to KRAS status.
METHODS: PubMed, EMBASE, Cochrane database and American Society of Clinical Oncology meeting abstracts were searched for randomized controlled trials (RCTs) reporting the effect of KRAS status on efficacy of chemotherapy regimen with or without cetuximab in mCRC. Baseline information such as sex and age was summarized from the included studies. Hazard ratios of progression-free survival (PFS) and overall survival (OS) as well as objective response based on KRAS status were extracted for analysis.
RESULTS: A total of 8 RCTs with 6780 patients were included. The combined analysis showed that cetuximab failed to improve the OS and PFS in patients with mCRC. However, in subgroup analysis, the pooled data showed that addition of cetuximab to irinotecan containing chemotherapy regimen was sufficient to improve OS and PFS in wild-type KRAS mCRC patients, but not in patients with mutant-type KRAS. The addition of cetuximab increased the incidence of adverse events such as diarrhea, rash, skin toxicity/rash, and nausea and vomiting. There was no significant publication bias existing in the included studies.
CONCLUSION: The clinical benefit of cetuximab was only confirmed in patients with wild-type KRAS. KRAS status could be considered a biomarker of efficacy of cetuximab.
Core tip: The addition of cetuximab to irinotecan containing chemotherapy regimen was sufficient to improve overall survival and progression-free survival in wild-type KRAS metastatic colorectal cancer patients, but not in patients with mutant-type KRAS.
- Citation: Li XX, Liang L, Huang LY, Cai SJ. Standard chemotherapy with cetuximab for treatment of colorectal cancer. World J Gastroenterol 2015; 21(22): 7022-7035
- URL: https://www.wjgnet.com/1007-9327/full/v21/i22/7022.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i22.7022
Colorectal cancer remains one of the most common cancers worldwide and its incidence was about 1.2 million in 2008[1]. In the past few years, progress has been achieved in improving outcome of metastatic colorectal cancer (mCRC), and this was mainly due to the application of novel molecular targeted agents[2,3]. However, recent evidence[4-6] showed that addition of cetuximab to chemotherapy did not improve the outcome for patients with mCRC, making the anti-tumor effect of cetuximab controversial, and indicating that cetuximab should be recommended based on individual information. Therefore, it is urgent to identify patients who could benefit from cetuximab treatment most and this relies on effective biomarkers in predicting efficacy of cetuximab in the treatment of mCRC.
Cetuximab is an IgG1 monoclonal antibody to the epidermal growth factor receptor (EGFR) and it exerts clinical activity in mCRC patients who are chemotherapy-resistant[6-8]. A phase III trial in patients with oxaliplatin and fluoropyrimidines-refractory mCRC who were randomized to cetuximab plus irinotecan showed an improved outcome for the addition of cetuximab[9]. Cetuximab has been approved by United States Food and Drug Administration in 2004. However, not all the individuals are sensitive to cetuximab, and investigations about influencing factors of its effectiveness have emerged, with one of the best known being KRAS status[10].
The KRAS protein is one of the most important downstream effectors coupling EGFR to intracellular signaling cascades, leading to cell growth, division, motility, and inhibition of apoptosis[11,12]. Single-nucleotide point mutations in the KRAS gene are found in approximately 40% of patients with metastatic CRC, including mutations in codons 12 and 13 of exon 2[12,13]. These mutations of KRAS may contribute to the lack of response to anti-EGFR monoclonal antibodies in patients with mCRC[10-12]. A meta-analysis[14] of pooled data from the CRYSTAL[15] and OPUS[16] studies confirmed that in patients with KRAS wild-type tumours, adding cetuximab to chemotherapy led to a significant improvement in overall survival (OS), progression-free survival (PFS) and overall response rate (ORR). However, other trials demonstrated that KRAS status was not predictive of benefit when adding cetuximab to the first-line therapy[12,17,18]. Thus, it is essential to evaluate whether KRAS is a biomarker of effectiveness of cetuximab using pooled data.
In regard of issues mentioned above, the present meta-analysis was to investigate whether addition of cetuximab could improve treatment outcomes such as PFS and OS based on KRAS status in patients with mCRC, and whether KRAS status could be a useful indicator of benefit from cetuximab treatment.
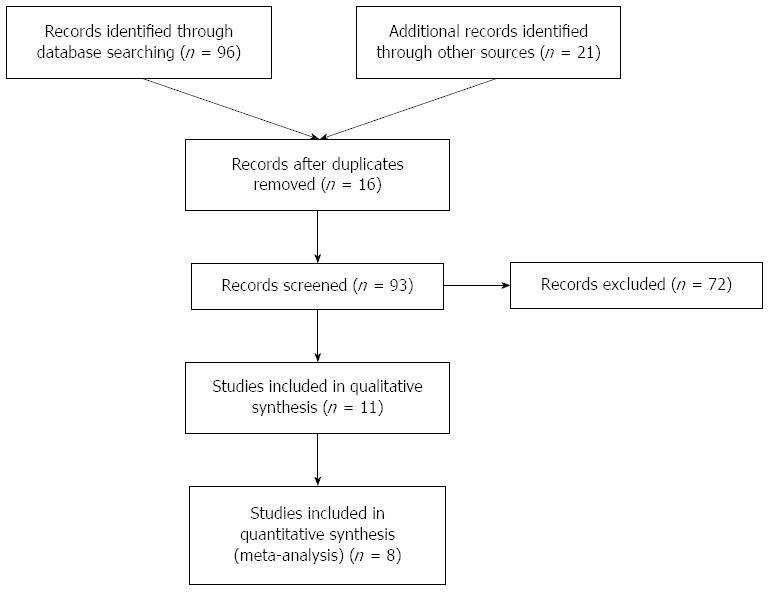
Population, intervention, control, and outcome (PICO) were defined prior to literature research. Then, electronic databases comprising PubMed, EMBASE, Cochrane, and American Society of Clinical Oncology meeting abstract (conference on colorectal cancer) were selected and used to search for randomized controlled trials (RCTs) comparing chemotherapy regimen with or without cetuximab in treatment of mCRC based on KRAS status. The search terms used were: [“colorectal neoplasms/therapy”(Mesh) or “carcinoma, colorectal “ or “tumor, colorectal”] and (“cetuximab” or “erbitux” or “MAb C225” or “anti-EGFR agents”) and (“stage III” or “stage IV” or “metasta?” or “advanced”) and (“KRAS” or “K-ras”). We also used a manual reference search for relevant articles, including original articles and reviews, to identify additional studies. If more than one article was published using the same case series, only the study with the latest data was included. The search was restricted to published English language papers. The literature search was updated on December 31, 2013. The detailed information of the search strategy for the eligible studies is presented in flow diagram provided by PRISMA (Figure 1).
Inclusion criteria were: (1) high quality RCTs performed in mCRC patients, either in form of a full article or a meeting abstract; (2) mCRC patients treated with traditional chemotherapy regimen with or without cetuximab; (3) RCTs comparing cetuximab + chemotherapy vs chemotherapy only, with regard to KRAS status; and (4) primary endpoints were PFS and/or OS, and secondary endpoints were OR and toxicity information.
Information in each eligible study was carefully extracted and identified by two reviewers independently (Li XX and Liang L), and classical data collection methods were applied during extraction process. The following data were extracted from the included studies: numbers of patients enrolled, publication date, characteristics of patients such as age and gender, and other data such as clinical stage, method of randomization, chemotherapy regimen and details of first-line chemotherapy, doses of cetuximab, PFS, OS, and OR. If hazard ratio (HR) and its variance were not available directly from original article, the method of Parmar et al[19] was introduced to establish estimates of these information. For identification of each eligible study, the first author’ name and publication year were used.
The protocols of GRADE were used to evaluate the quality of each RCT included for this meta-analysis. Quality control was performed independently by two reviewers. If there was a disagreement about quality of a certain study, another reviewer was involved to solve it. Funnel plots were also introduced to assess the publication bias.
RevMan 5.2 software which was provided by the Cochrane Collaboration was applied to perform all of the statistical analyses, and introduction of the Cochrane Collaboration for meta-analysis was followed to ensure the accuracy of whole analysis process. We assessed the between-study heterogeneity by Cochran’s Q test and quantified by I2 (a significance level of P < 0.10 and/or I2≥ 50%). If the P-value of the Q test is > 0.05, the summary OR estimate of each study was calculated using the fixed-effect model. Otherwise, the random-effect model was used. A funnel plot and Egger’s linear regression test were used to investigate any possible publication bias[20]. For all analyses, a two-sided P-value less than 0.05 was considered to be statistically significant.
The statistical methods of this study were reviewed by San-Jun Cai from Department of Colorectal Surgery, Fudan University Shanghai Cancer Center.
A total of eight RCTs[17,21-27] were included for this meta-analysis involving a number of 6780 mCRC patients. Among these eligible trials, full articles are available from databases. The baseline information and adverse events of these studies are shown in Tables 1 and 2. Five of them[17,22,25-27] assessed oxaliplatin based chemotherapy regimen plus cetuximab in the first-line treatment of metastatic or advanced CRC, while two studies[21,24] evaluated the effect of cetuximab in combination with the FOLFIRI regimen on outcome of metastatic or advanced CRC patients, and only one trial[23] involved both FOLFIRI or oxaliplatin based regimen. All studies[17,21-26] reported the status of KRAS in mCRC except the study of Borner et al[27]. Data from these RCTs were sufficient to support the statistically pooled analysis of PFS and OS.
| Study | Type of article | Patients | Intervention | Main endpoints | Mutation status reported | Quality control | ||
| Cetuximab + chemotherapy | Chemotherapy | (HR, 95%CI) | KRAS | BRAF | ||||
| Borner et al[27] | Full manuscript | 74 | Cetuximab + XELOX | XELOX | PFS: NR; OS: NR | No | No | Moderate |
| Bokemeyer et al[25] | Full manuscript | 337 | Cetuximab + FOLFOX-4 | FOLFOX-4 | PFS: 0.931, 0.705-1.230; | Yes | Yes | Good |
| OS: 1.015, 0.791-1.303 | ||||||||
| Van Cutsem et al[21] | Full manuscript | 1198 | Cetuximab + FOLFIRI | FOLFIRI | PFS: 0.851, 0.726-0.998; | Yes | Yes | Good |
| OS: 0.878, 0.774-0.995 | ||||||||
| Maughan et al[17] | Full manuscript | 1630 | Cetuximab + oxaliplatin + fluoropyrimidine | Oxaliplatin + fluoropyrimidine | PFS: 0.96, 0.82-1.12; | Yes | Yes | Good |
| OS: 1.04, 0.87-1.23 | ||||||||
| Tveit et al[22] | Full manuscript | 571 | Cetuximab + FLOX | FLOX | PFS: NR; OS: NR | Yes | Yes | Good |
| Alberts et al[26] | Full manuscript | 2686 | Cetuximab + mFOLFOX6 | mFOLFOX6 | PFS: NR; OS: NR | Yes | Yes | Good |
| Huang et al[24] | Full manuscript | 146 | Cetuximab + FOLFIRI | FOLFIRI | PFS: 0.53, 0.26-1.10; | Yes | Yes | Good |
| OS: 0.45, 0.2-1.2 | ||||||||
| Ye et al[23] | Full manuscript | 138 | Cetuximab + mFOLFOX6/ FOLFIRI | mFOLFOX6/ | PFS: 0.60, 0.41-0.87; | Yes | Yes | Good |
| FOLFIRI | OS: 0.54, 0.33-0.89 | |||||||
| Study | Neutropenia | Nausea and vomiting | Skin toxicity/rash | Rash | Diarrhea | |||||
| Control group | Cetuximab group | Control group | Cetuximab group | Control group | Cetuximab group | Control group | Cetuximab group | Control group | Cetuximab group | |
| Borner et al[27] | 3 | 0 | 6 | 10 | 0 | 16 | 0 | 8 | 16 | 22 |
| Bokemeyer et al[25] | 57 | 51 | NA | NA | 1 | 30 | 1 | 19 | 12 | 14 |
| Van Cutsem et al[21] | 150 | 169 | 30 | 28 | 1 | 117 | 0 | 49 | 63 | 94 |
| Maughan et al[17] | NA | NA | NA | NA | 14 | 114 | NA | NA | NA | NA |
| Tveit et al[22] | 47 | 95 | 3 | 14 | 1 | 51 | 1 | 51 | 10 | 33 |
| Alberts et al[26] | 89 | 110 | 59 | 70 | NA | NA | 3 | 186 | 83 | 148 |
| Huang et al[24] | 15 | 3 | 0 | 18 | NA | NA | 0 | 11 | 15 | 6 |
| Ye et al[23] | 6 | 8 | 3 | 3 | 1 | 2 | 2 | 9 | 3 | 4 |
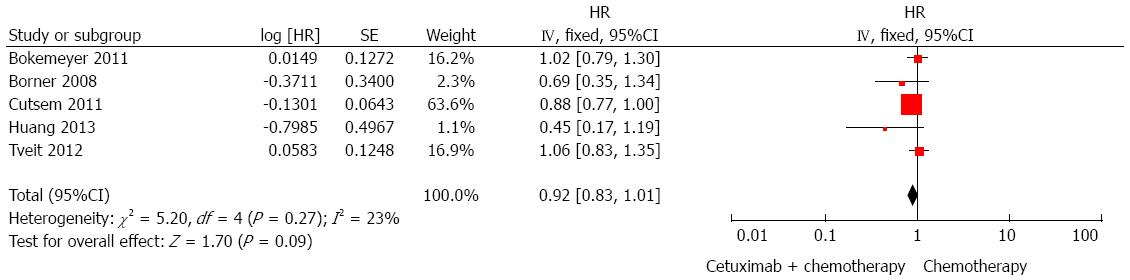
OS regardless of KRAS status: Five RCTs[21,22,24,25,27] were included for the analysis of whether addition of cetuximab to standard chemotherapy could improve OS than chemotherapy alone. The result showed that the application of cetuximab failed to provide a significant improvement of OS regardless of KRAS status (HR = 0.92, 95%CI: 0.83-1.01; P > 0.05; Figure 2).
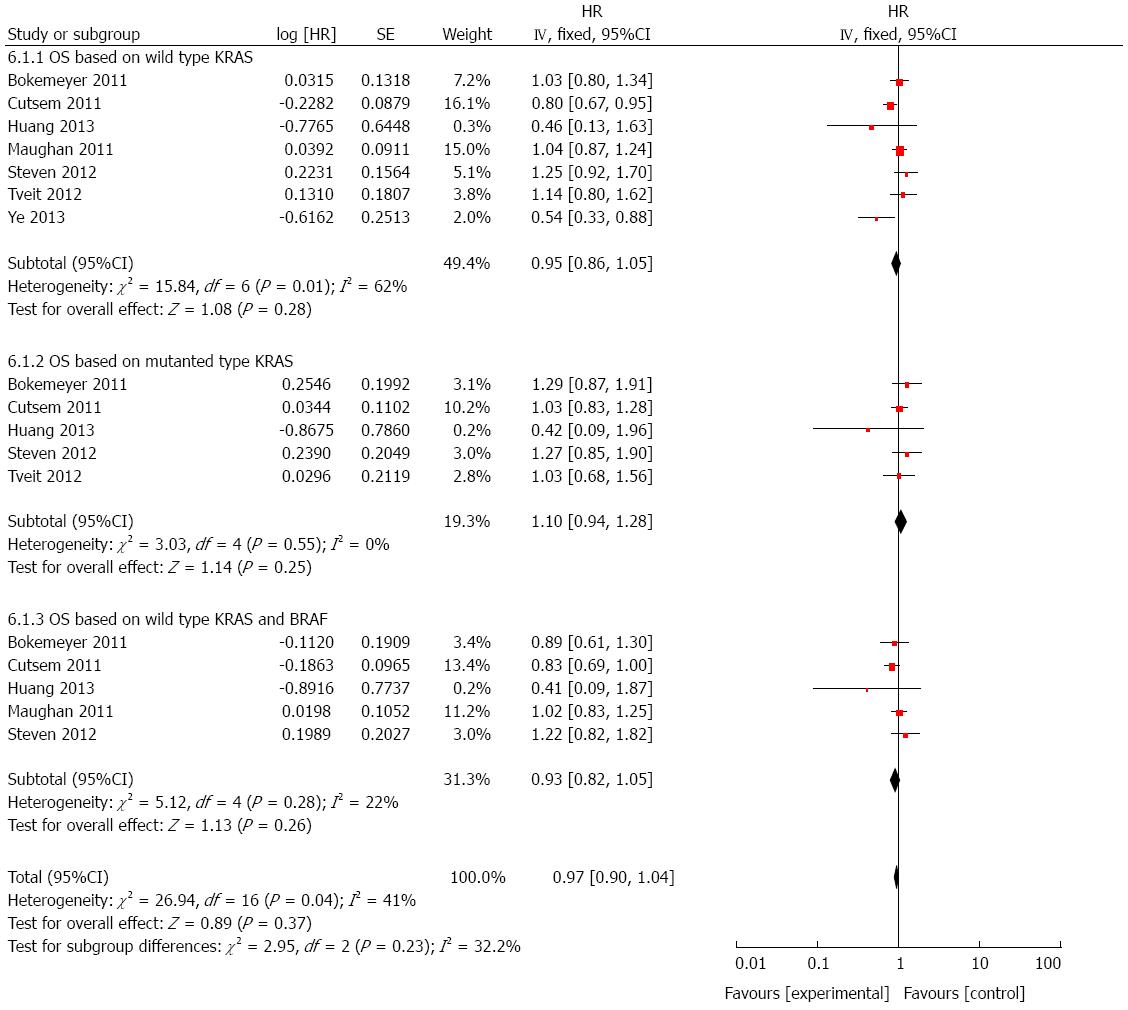
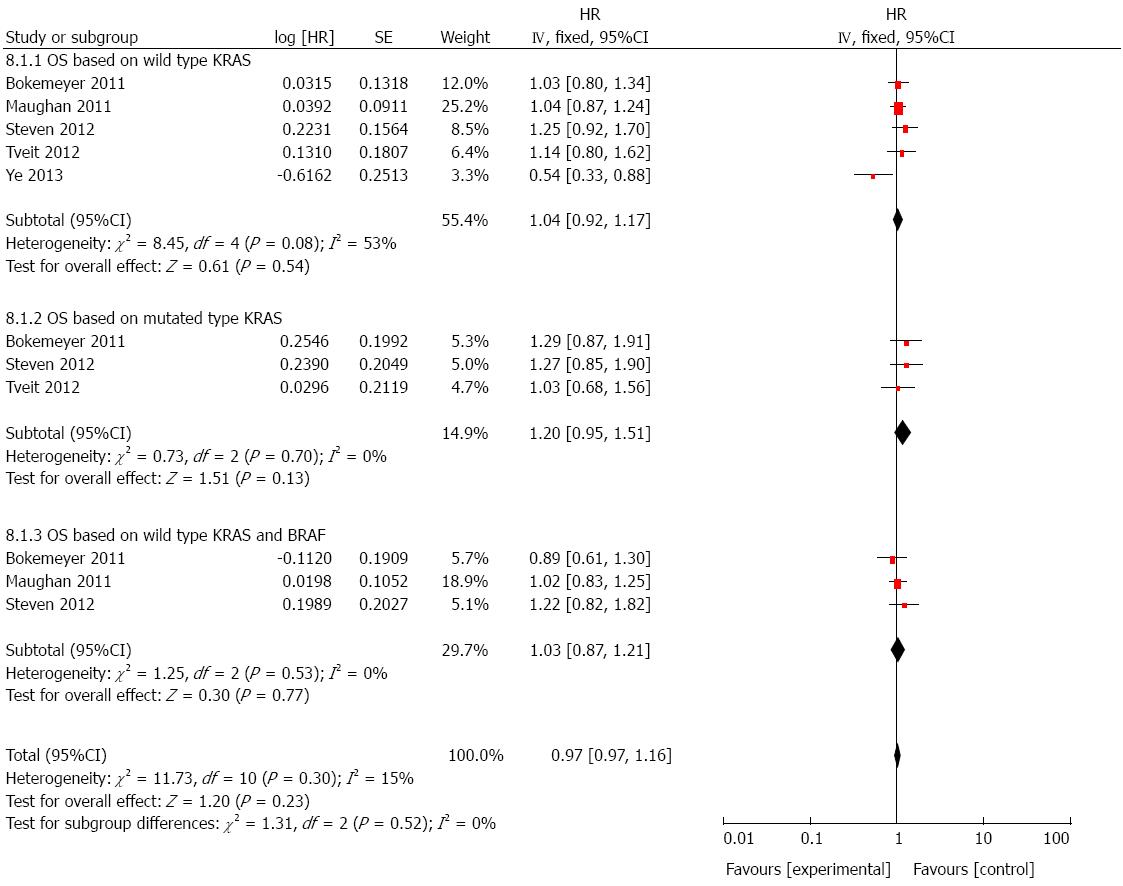
OS based on wild-type KRAS: To evaluate whether cetuximab plus chemotherapy could benefit OS in population harboring wild-type KRAS, seven studies were included[17,21-26]. As shown in Figure 3, though cetuximab plus chemotherapy seemed to provide a benefit in prolonging OS, there was no statistically significance (HR = 0.95, 95%CI: 0.86-1.05; P > 0.05).
OS based on mutated KRAS: There was five studies[21,22,24-26] involved in the analysis of OS based on mutant KRAS in patients who received cetuximab combined with chemotherapy. A significant difference was not observed from the pooled analysis (HR = 1.10, 95%CI: 0.94-1.28; P > 0.05; Figure 3).
OS based on wild-type KRAS and BRAF: We also analyzed the effect of cetuximab on OS in patients with both wild-type KRAS and BRAF by using five studies[21,22,24-26]. Still, it showed that there was no significant improvement on OS (HR = 0.93, 95%CI: 0.82-1.05; P > 0.05; Figure 3), though in the setting of wild-type targeted genes.
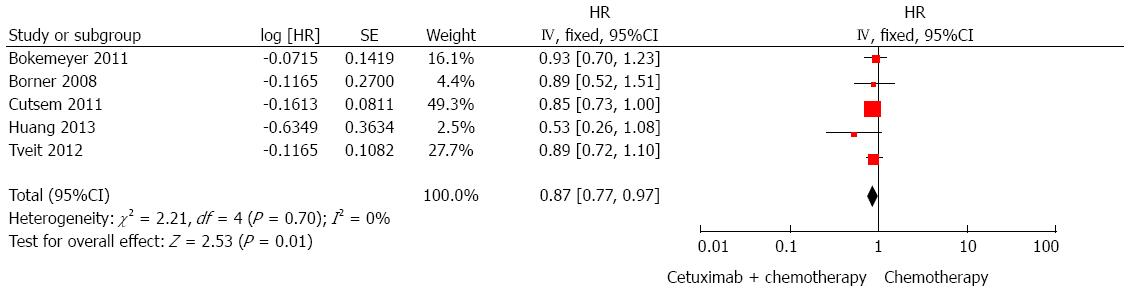
PFS regardless of KRAS status: Five trials[21,22,24,25,27] were used to evaluate the improvement in PFS with cetuximab combined with chemotherapy vs chemotherapy alone. Compared with chemotherapy, there was a significantly prolonged PFS in patients treated with cetuximab (HR = 0.87, 95%CI: 0.77-0.97; P < 0.05; Figure 4).
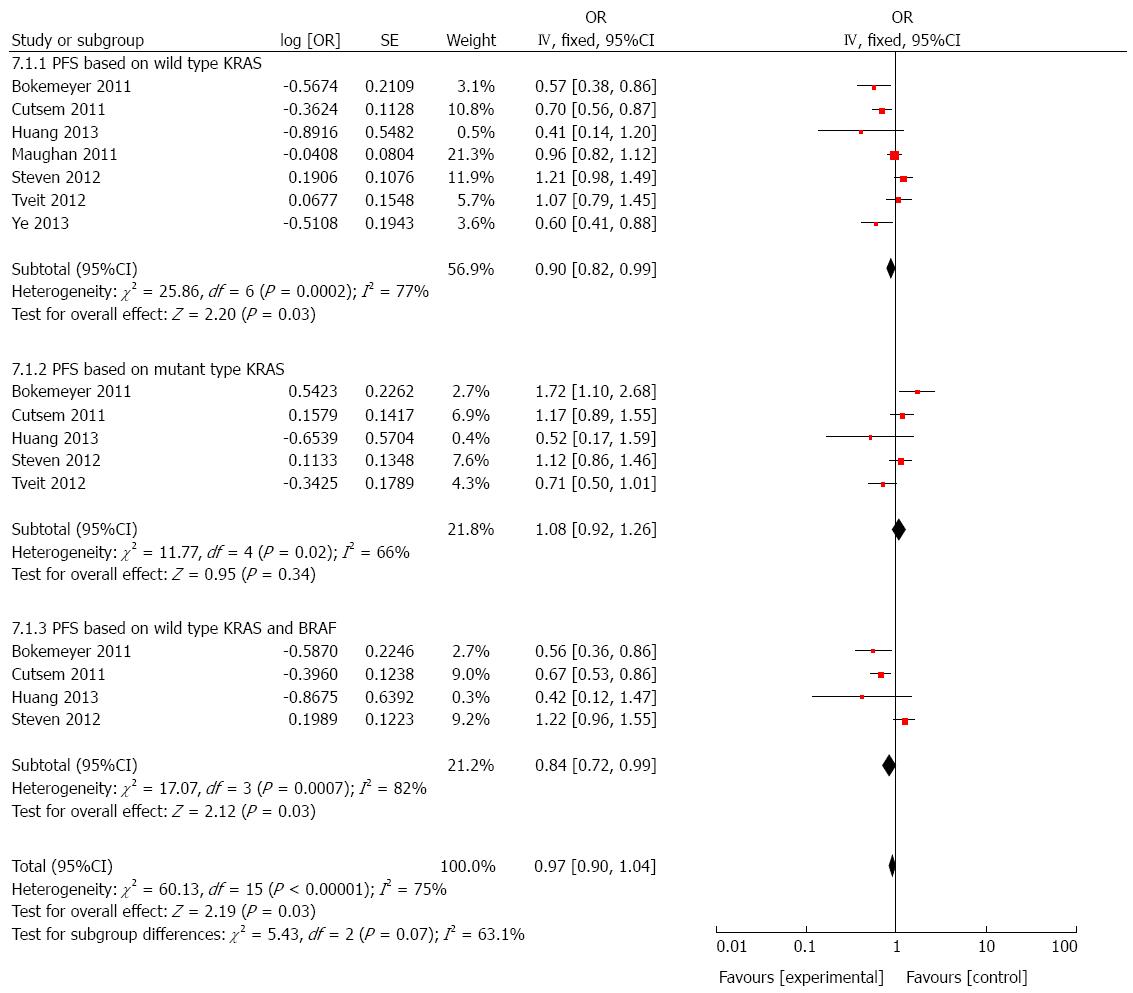
PFS based on wild-type KRAS: We further performed a sub-group analysis of cetuximab combined with chemotherapy vs chemotherapy in patients having wild-type KRAS. As shown in Figure 5, cetuximab succeeded to provide a significant improvement in PFS (HR = 0.90, 95%CI: 0.82-0.99; P < 0.05).
PFS based on mutated KRAS: A total of five studies[21,22,24-26] were selected for the analysis of PFS based on mutant KRAS in patients who received cetuximab combined with chemotherapy. A significant difference was not observed from the result (HR = 1.08, 95%CI: 0.92-1.26; P > 0.05, Figure 5).
PFS based on wild-type KRAS and BRAF: Analysis of cetuximab plus chemotherapy vs chemotherapy was performed using data extracted from four RCTs[21,24-26]. A positive result was obtained and it presented that cetuximab therapy benefited PFS significantly in patients with wild-type KRAS and BRAF (HR = 0.84, 95%CI: 0.72-0.99; P < 0.05; Figure 5).
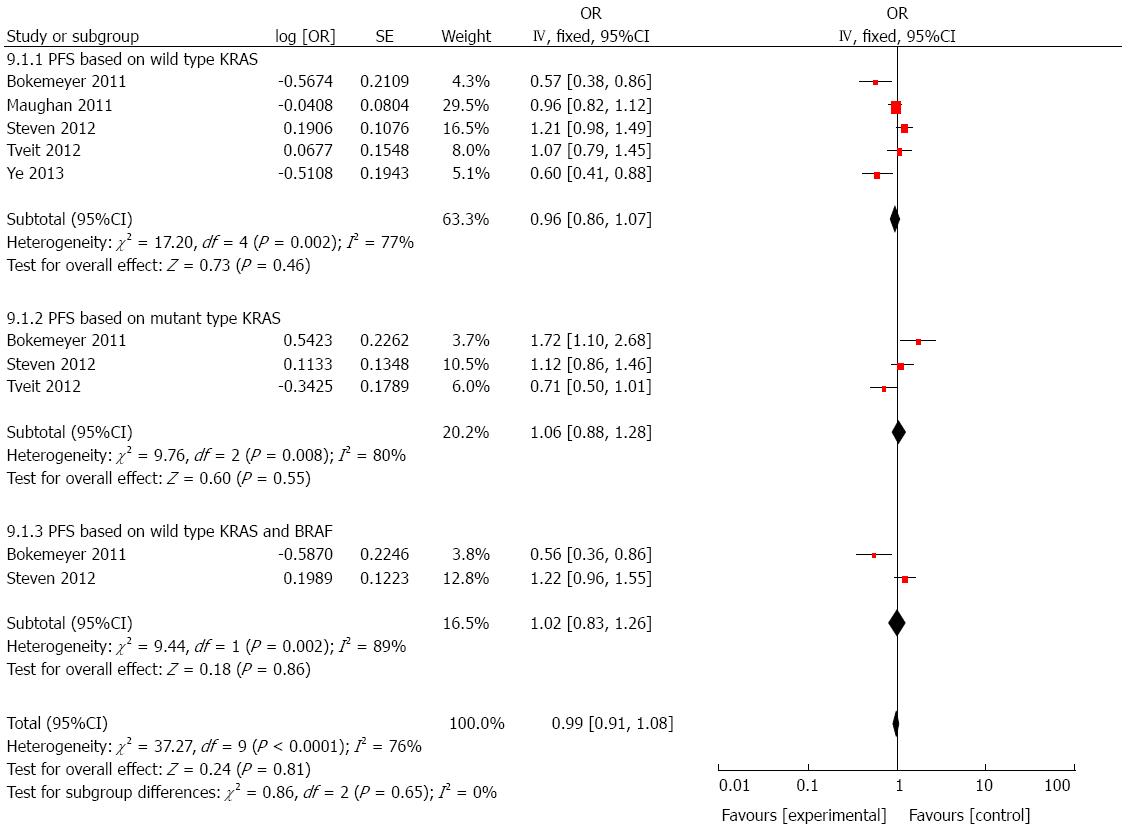
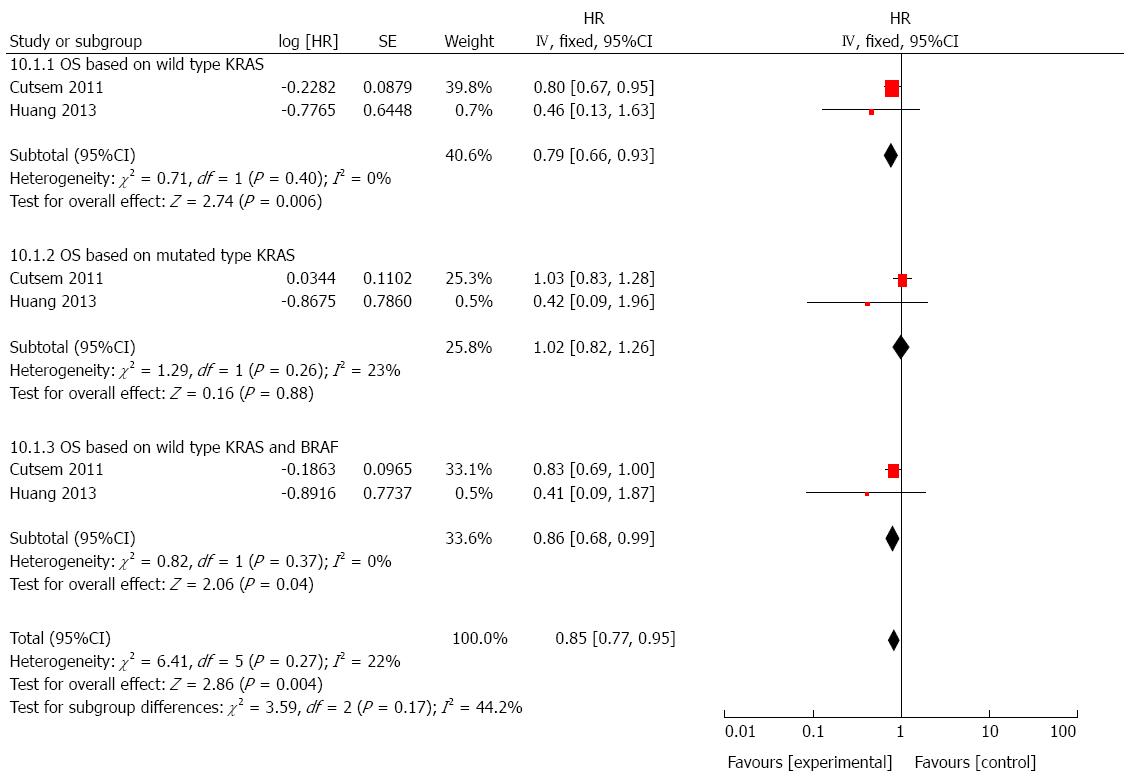
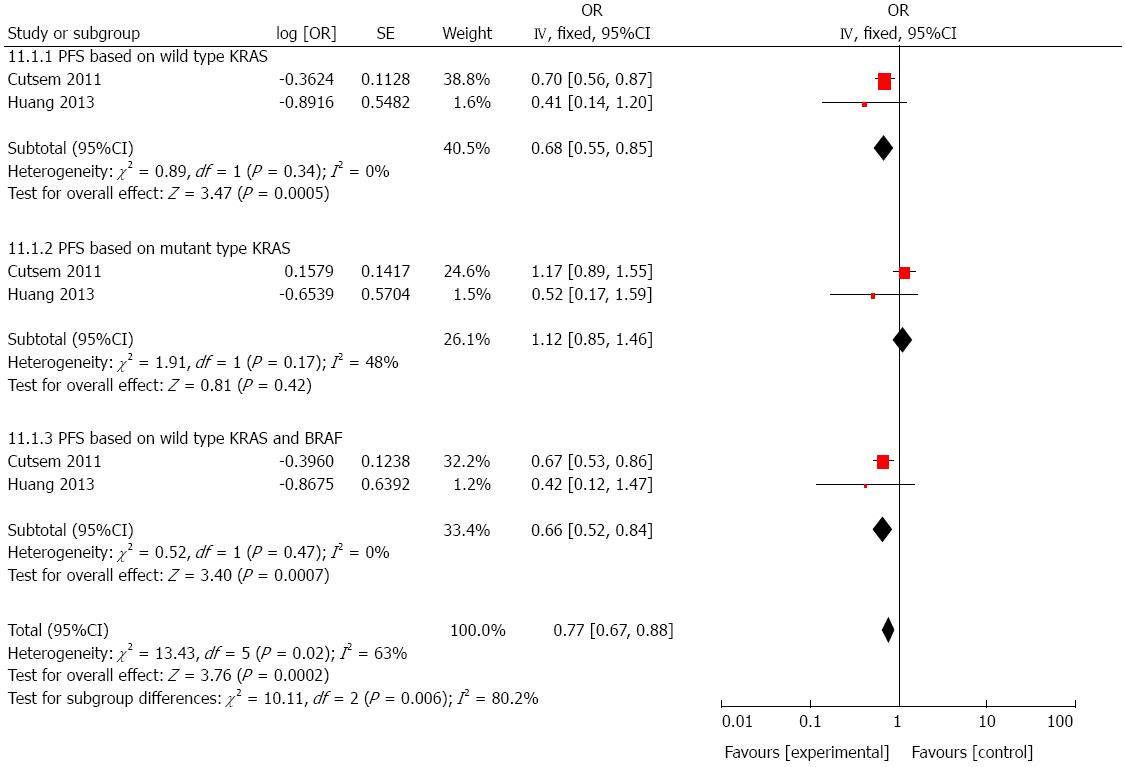
We performed another combined analysis based on different chemotherapy regimens. Whether the regimen contained irinotecan was used as the standard to group the included studies. Cetuximab added to irinotecan-free regimen did not significantly improve the OS (HR = 1.06, 95%CI: 0.97-1.16; P = 0.23; Figure 6) or PFS (HR = 0.99, 95%CI: 0.91-1.08; P = 0.81; Figure 7) in patients with mCRC, regardless of status of KRAS and/or BRAF. Next, we compared the outcomes of patients receiving cetuximab and irinotecan, and the weighted results showed that cetuximab and irinotecan significantly improved OS (HR = 0.85, 95%CI: 0.77-0.95; P = 0.004; Figure 8) and PFS (HR = 0.77, 95%CI: 0.67-0.88; P = 0.0002; Figure 9) in mCRC patients with wild-type KRAS, but not in patients with mutant KRAS.
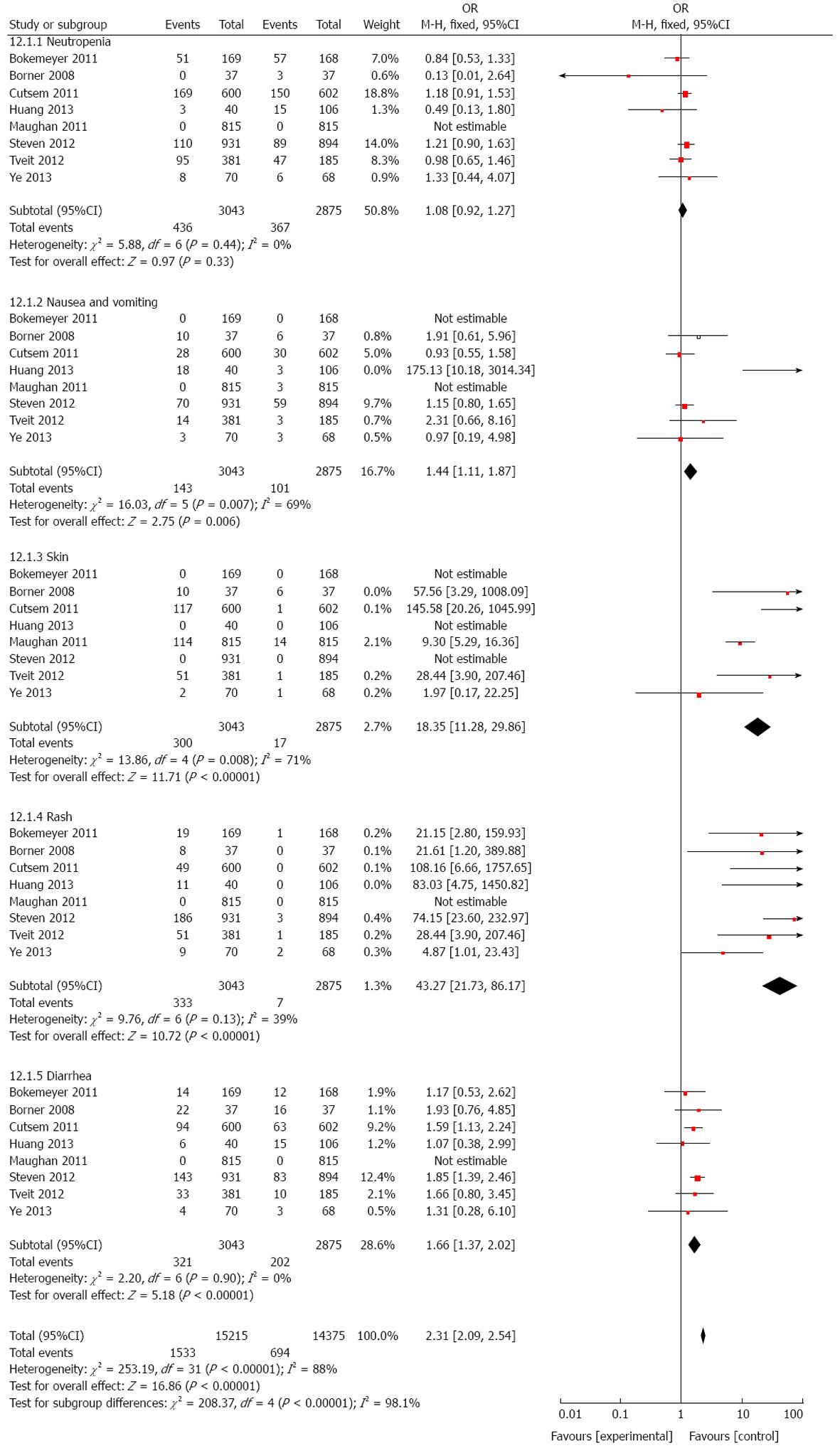
As cetuximab is a targeted agent, we examined the effect of cetuximab on adverse events. The results determined that patients who received cetuximab suffered from more adverse events such as skin toxicity/rash (HR = 18.35, 95%CI: 11.28-29.86; P = 0.008, Figure 10), rash (HR = 43.27, 95%CI: 21.73-86.17; P = 0.0002; Figure 10), diarrhea (HR = 1.66, 95%CI: 1.37-2.02; P < 0.001; Figure 10), and nausea and vomiting (HR = 1.44, 95%CI: 1.11-1.87; P = 0.007; Figure 10), indicating that the application of cetuximab should be carefully considered not only based on status of targeted genes, but also the quality of life and safety.
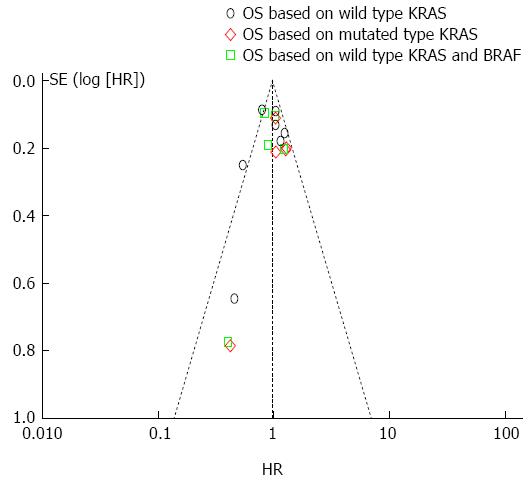
To evaluate the publication bias, we performed the Egger’s test and funnel plot. As illustrated by Figure 11, only 2 studies exceeded the confidence interval and the Egger’s test showed that there was no significant publication bias within the included studies (P < 0.05).
Cetuximab, in combination with chemotherapy, has been approved for the treatment of mCRC patients[28]. Many clinical trials[14-18,28] have been published to evaluate the efficacy of cetuximab in mCRC, especially based on KRAS status. However, the outcomes from these studies were not consistent. Thus, it is essential to provide clinical evidence relating to the application of cetuximab in mCRC treatment. Indeed, several meta-analyses[29-33] have been published in recent years, but the arguments about whether cetuximab could benefit outcomes of mCRC patients with different KRAS status still exist. The present meta-analysis was performed to address the issues mentioned above, and to increase the statistical power of efficacy analysis of cetuximab in mCRC patients and further to identify what kind of population could benefit from treatment of cetuximab most.
The present meta-analysis confirms that adding anti-EGFR therapy to standard chemotherapy could result in clinical benefits in the treatment of mCRC containing wild-type KRAS, with a significantly prolonged PFS. For patients harboring wild-type KRAS, a statistically significant longer PFS was found when using cetuximab with traditional chemotherapy regimens. However, cetuximab treatment was associated with an impaired improvement in OS, and no statistical significance was achieved. Compared with the clinical benefit of addition of cetuximab to chemotherapy regimens in wild-type KRAS patients, mutation of KRAS is a predictor of less sensitivity to cetuximab in mCRC patients with regard to PFS and OS. The subgroup analysis demonstrated that cetuximab in combination with irinotecan containing regimen could improve OS and PFS in patients with wild-type KRAS and/or BRAF, but not in patients with mutant KRAS. These benefits were not observed in patients treated with irinotecan-free chemotherapy. Notably, the incidence of adverse events in the cetuximab group was much higher than that in patients without cetuximab treatment.
The results of this meta-analysis is in accordance with those of other meta-analyses[34]. In the study performed by Qiu et al[31], they compared the efficacy of cetuximab combined with chemotherapy vs chemotherapy for patients with mCRC, as well as the influence of KRAS mutation status on the outcomes, and the results showed that in wild-type KRAS patients, cetuximab plus chemotherapy significantly improved PFS when compared with chemotherapy alone, but not for OS, whereas in mutant KRAS patients, there was no significant benefit between those treated with cetuximab plus chemotherapy and those with chemotherapy alone regarding PFS and OS. In addition, Bokemeyer et al[14] enrolled CRYSTAL and OPUS studies for meta-analysis and by analyzing pooled data from the CRYSTAL and OPUS studies, they confirmed that adding cetuximab to first-line chemotherapy in patients with KRAS wild-type mCRC could obtain benefit in all efficacy end-points. Barni et al[32] conducted a meta-analysis evaluating the efficacy of cetuximab in patients with wild-type KRAS as second- or further-line therapy, and they demonstrated that treatment with cetuximab plus chemotherapy in mCRC patients with wild-type KRAS pretreated with one or more lines of therapy could improve survival outcomes, however, this meta-analysis did not include patients treated with chemotherapy alone and patients with mutant KRAS status.
In contrast to the results mentioned above, the meta-analysis of Zhou et al[29] showed that the addition of cetuximab or panitumumab to oxaliplatin-based chemotherapy in first-line treatment of mCRC in wild-type KRAS population did not improve survival benefit or response rate. They explained that the nature and interaction of drugs used in combination may be responsible for this observation[29]. Indeed, constitutive activation of the intracellular signaling pathway downstream of EGFR would counteract the effects of anti-EGFR agents[12], though it is in the setting of KRAS mutation status. In addition, another meta-analysis demonstrated that efficacy of cetuximab could be influenced by drugs used in combination[34]. Indeed, irinotecan has been widely used in the treatment of mCRC, but not all of the patients were treated with this agent. Our study proved that efficacy of cetuximab combined with irinotecan chemotherapy was much better those without irinotecan. These all demonstrated the complexity of tumor pathology and capacity of response to different chemotherapy. More elegant trials considering suitable drugs used in combination treatment are needed.
It seems that KRAS status is a good predictor of sensitivity to cetuximab treatment, making it reasonable to detect the exact mutation location in KRAS gene. In our study, we observed that patients with wild-type KRAS could benefit a lot from cetuximab treatment, while this did not happen in patients with mutant KRAS. However, several studies[30,35,36] reported that certain specific mutations in KRAS could gain a greater clinical response to anti-EGFR treatment than patients with other KRAS mutations. This again demonstrated the complexity in the treatment of mCRC patients regarding KRAS status.
There are a few limitations in the present meta-analysis. First, the randomization is not appropriately applied in some of the studies, and heterogeneity in trial protocols, age, sex, and endpoint variables is inevitable. Second, information about PFS and OS is not directly available from each included study. Finally, only a subset of specimens were available from all participants, despite that the initial trials were carefully designed. However, these limitations could be attenuated partly by using random effect model analysis. More elegant RCTs assessing efficacy of cetuximab in the treatment of mCRC patients with different KRAS status are warranted.
In conclusion, the results from this meta-analysis strength the evidence supporting the use of cetuximab treatment in combination with traditional chemotherapy in mCRC patients with wild-type KRAS. KRAS status should be explored prior to the initiation of adding cetuximab to treatment of mCRC patients in order to avoid ineffective and toxic therapies. For patients with unclear status of KRAS and/or BRAF, cetuximab should be initially considered. More challenges emerged in the search of better biomarkers of cetuximab in mCRC, in the setting that certain KRAS mutational status is also associated with an favorable outcome when encountering with other mutational status. It is expected to find that judicious application of biomarkers will provide more chances to optimize the use of cetuximab.
The application of novel molecular targeted agents prolongs the survival of patients with metastatic colorectal cancer (mCRC). However, some research showed that addition of cetuximab to chemotherapy did not improve the outcome of mCRC patients, making the anti-tumor effect of cetuximab controversial, and indicating that cetuximab should be recommended based on individual information. Therefore, it is urgent to identify patients who could benefit from cetuximab treatment most and this relies on effective biomarkers in predicting efficacy of cetuximab in the treatment of mCRC.
The KRAS protein is one of the most important downstream effectors coupling EGFR to intracellular signaling cascades. The mutations of KRAS may contribute to the lack of response to anti-EGFR monoclonal antibodies in patients with mCRC. Thus, it is essential to evaluate whether KRAS is a biomarker of effectiveness of cetuximab using pooled data.
Pooled data from the CRYSTAL and OPUS studies confirmed that in patients with KRAS wild-type tumors, adding cetuximab to chemotherapy led to a significant improvement in overall survival (OS), progression-free survival (PFS) and overall response rate. However, other trials demonstrated that KRAS status was not predictive of benefit when adding cetuximab to the first-line therapy.
The results from this meta-analysis strength the evidence supporting the use of cetuximab treatment in combination with traditional chemotherapy in mCRC patients with wild-type KRAS. KRAS status should be explored prior to the initiation of adding cetuximab to treatment of mCRC patients in order to avoid ineffective and toxic therapies. For patients with unclear status of KRAS and/or BRAF, cetuximab should be initially considered.
In this systematic review, the authors performed a series of subgroup analysis regarding not only FPS or OS of the patients, but also the mutation status of KRAS and/or BRAF, to compare the efficacy and safety of additional cetuximab to irinotecan containing chemotherapy under these different circumstances.
This is a good systematic review in which the authors analyzed the cetuximab treatment in mCRC with regard to KRAS status. The results are clear and interesting and suggest that KRAS is a biomarker of effectiveness of cetuximab, and KRAS status should be explored prior to the initiation of adding cetuximab to treatment of mCRC patients in order to avoid ineffective and toxic therapies.
P- Reviewer: Chan A, Wittmann T, Zhou LM S- Editor: Yu J L- Editor: Wang TQ E- Editor: Liu XM
| 1. | World Health Organisation. Globocan. 2008;. [Cited in This Article: ] |
| 2. | Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, Labianca R, Seitz JF, O’Callaghan CJ, Francini G. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664-8670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 491] [Cited by in F6Publishing: 504] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 3. | Arnold D, Stein A. New developments in the second-line treatment of metastatic colorectal cancer: potential place in therapy. Drugs. 2013;73:883-891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Woo J, Palmisiano N, Tester W, Leighton JC. Controversies in antiepidermal growth factor receptor therapy in metastatic colorectal cancer. Cancer. 2013;119:1941-1950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ, Sinnige HA. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1000] [Cited by in F6Publishing: 977] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 6. | Troiani T, Zappavigna S, Martinelli E, Addeo SR, Stiuso P, Ciardiello F, Caraglia M. Optimizing treatment of metastatic colorectal cancer patients with anti-EGFR antibodies: overcoming the mechanisms of cancer cell resistance. Expert Opin Biol Ther. 2013;13:241-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3767] [Cited by in F6Publishing: 3625] [Article Influence: 181.3] [Reference Citation Analysis (0)] |
| 8. | Lenz HJ, Van Cutsem E, Khambata-Ford S, Mayer RJ, Gold P, Stella P, Mirtsching B, Cohn AL, Pippas AW, Azarnia N. Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J Clin Oncol. 2006;24:4914-4921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 427] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 9. | Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311-2319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 684] [Cited by in F6Publishing: 694] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 10. | Oyan B. Why do targeted agents not work in the adjuvant setting in colon cancer? Expert Rev Anticancer Ther. 2012;12:1337-1345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1186] [Cited by in F6Publishing: 1142] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 12. | Patel GS, Karapetis CS. Personalized treatment for advanced colorectal cancer: KRAS and beyond. Cancer Manag Res. 2013;5:387-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682-4689. [PubMed] [Cited in This Article: ] |
| 14. | Bokemeyer C, Van Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, Celik I, Köhne CH. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48:1466-1475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 417] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 15. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2901] [Cited by in F6Publishing: 3021] [Article Influence: 201.4] [Reference Citation Analysis (1)] |
| 16. | Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1218] [Cited by in F6Publishing: 1202] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 17. | Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103-2114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 702] [Cited by in F6Publishing: 731] [Article Influence: 56.2] [Reference Citation Analysis (2)] |
| 18. | Tveit K, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, Kure E, Ikdahl T, Skovlund E, Christoffersen T. Randomized phase III study of 5-fluorouracil/folinate/oxaliplatin given continuously or intermittently with or without cetuximab, as first-line treatment of metastatic colorectal cancer: The NORDIC VII study (NCT00145314), by the Nordic Colorectal Cancer Biomodulation Group. Ann Oncol. 2010;21 Suppl 8:viii9. [Cited in This Article: ] |
| 19. | Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815-2834. [PubMed] [Cited in This Article: ] |
| 20. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] [Cited in This Article: ] |
| 21. | Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-2019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1314] [Cited by in F6Publishing: 1452] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 22. | Tveit KM, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, Sigurdsson F, Kure E, Ikdahl T, Skovlund E. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol. 2012;30:1755-1762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 407] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 23. | Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, Ye QH, Yu Y, Xu B, Qin XY. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol. 2013;31:1931-1938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 297] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 24. | Huang J, Nair SG, Mahoney MR, Nelson GD, Shields AF, Chan E, Goldberg RM, Gill S, Kahlenberg MS, Quesenberry JT. Comparison of FOLFIRI with or without cetuximab in patients with resected stage III colon cancer; NCCTG (Alliance) intergroup trial N0147. Clin Colorectal Cancer. 2014;13:100-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, Celik I, Schlichting M, Koralewski P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535-1546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 532] [Cited by in F6Publishing: 586] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 26. | Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, Smyrk TC, Sinicrope FA, Chan E, Gill S. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 339] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 27. | Borner M, Koeberle D, Von Moos R, Saletti P, Rauch D, Hess V, Trojan A, Helbling D, Pestalozzi B, Caspar C. Adding cetuximab to capecitabine plus oxaliplatin (XELOX) in first-line treatment of metastatic colorectal cancer: a randomized phase II trial of the Swiss Group for Clinical Cancer Research SAKK. Ann Oncol. 2008;19:1288-1292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Chen MC, Chiang FF, Wang HM. Cetuximab plus chemotherapy as first-line treatment for metastatic colorectal cancer: effect of KRAS mutation on treatment efficacy in Taiwanese patients. Neoplasma. 2013;60:561-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Zhou SW, Huang YY, Wei Y, Jiang ZM, Zhang YD, Yang Q, Xie DR. No survival benefit from adding cetuximab or panitumumab to oxaliplatin-based chemotherapy in the first-line treatment of metastatic colorectal cancer in KRAS wild type patients: a meta-analysis. PLoS One. 2012;7:e50925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Mao C, Huang YF, Yang ZY, Zheng DY, Chen JZ, Tang JL. KRAS p.G13D mutation and codon 12 mutations are not created equal in predicting clinical outcomes of cetuximab in metastatic colorectal cancer: a systematic review and meta-analysis. Cancer. 2013;119:714-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Qiu LX, Mao C, Zhang J, Zhu XD, Liao RY, Xue K, Li J, Chen Q. Predictive and prognostic value of KRAS mutations in metastatic colorectal cancer patients treated with cetuximab: a meta-analysis of 22 studies. Eur J Cancer. 2010;46:2781-2787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Barni S, Ghilardi M, Borgonovo K, Cabiddu M, Zaniboni A, Petrelli F. Cetuximab/irinotecan-chemotherapy in KRAS wild-type pretreated metastatic colorectal cancer: a pooled analysis and review of literature. Rev Recent Clin Trials. 2013;8:101-109. [PubMed] [Cited in This Article: ] |
| 33. | Hoyle M, Crathorne L, Peters J, Jones-Hughes T, Cooper C, Napier M, Tappenden P, Hyde C. The clinical effectiveness and cost-effectiveness of cetuximab (mono- or combination chemotherapy), bevacizumab (combination with non-oxaliplatin chemotherapy) and panitumumab (monotherapy) for the treatment of metastatic colorectal cancer after first-line chemotherapy (review of technology appraisal No.150 and part review of technology appraisal No. 118): a systematic review and economic model. Health Technol Assess. 2013;17:1-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Ku GY, Haaland BA, de Lima Lopes G. Cetuximab in the first-line treatment of K-ras wild-type metastatic colorectal cancer: the choice and schedule of fluoropyrimidine matters. Cancer Chemother Pharmacol. 2012;70:231-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Chen J, Ye Y, Sun H, Shi G. Association between KRAS codon 13 mutations and clinical response to anti-EGFR treatment in patients with metastatic colorectal cancer: results from a meta-analysis. Cancer Chemother Pharmacol. 2013;71:265-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Modest DP, Brodowicz T, Stintzing S, Jung A, Neumann J, Laubender RP, Ocvirk J, Kurteva G, Papai Z, Knittelfelder R. Impact of the specific mutation in KRAS codon 12 mutated tumors on treatment efficacy in patients with metastatic colorectal cancer receiving cetuximab-based first-line therapy: a pooled analysis of three trials. Oncology. 2012;83:241-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |