Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.511
Peer-review started: May 12, 2014
First decision: June 18, 2014
Revised: June 20, 2014
Accepted: July 30, 2014
Article in press: July 30, 2014
Published online: January 14, 2015
AIM: To investigate the associations between miRNA-103 (miR-103) and insulin resistance and nonalcoholic fatty liver disease (NAFLD).
METHODS: Serum samples were collected from 50 NAFLD patients who were overweight or obese (NAFLD group) and from 30 healthy subjects who served as controls (normal control group). Quantitative polymerase chain reaction was used to detect expression of miR-103. Fasting plasma glucose, fasting insulin, and triglyceride (TG) levels were measured. Homeostasis model assessment was used to evaluate basal insulin resistance (HOMA-IR). Patient height and weight were measured to calculate body mass index (BMI).
RESULTS: Compared with the normal control group, higher serum levels of miR-103 were expressed in the NAFLD group (8.18 ± 0.73 vs 4.23 ± 0.81, P = 0.000). When P = 0.01 (bilateral), miR-103 was positively correlated with HOMA-IR (r = 0.881), TG (r = 0.774) and BMI (r = 0.878), respectively. miR-103, TG and BMI were all independent factors for HOMA-IR (β = 0.438/0.657/0.251, P = 0.000/0.007/0.001). miR-103, TG, BMI and HOMA-IR were all risk factors for NAFLD (odds ratio = 2.411/16.196/1.574/19.11, P = 0.009/0.022/0.01/0.014).
CONCLUSION: miR-103 is involved in insulin resistance and NAFLD, and may be a molecular link between insulin resistance and NAFLD and a therapeutic target for these disorders.
Core tip: Insulin resistance activates development of nonalcoholic fatty liver disease (NAFLD), however, the molecular mechanism is not fully understood. We determined fasting plasma glucose, fasting insulin, triglyceride (TG) and the levels of miRNA-103 (miR-103) in the serum of patients with NAFLD. We found that higher levels of miR-103 were expressed in the serum of patients with NAFLD. miR-103 was positively correlated with homeostasis model assessment and was used to evaluate basal insulin resistance (HOMA-IR), TG and BMI, respectively. miR-103 was an independent factor for HOMA-IR and a risk factor for NAFLD. We conclude that miR-103 is involved in insulin resistance and NAFLD.
- Citation: Xu Q, Li Y, Shang YF, Wang HL, Yao MX. miRNA-103: Molecular link between insulin resistance and nonalcoholic fatty liver disease. World J Gastroenterol 2015; 21(2): 511-516
- URL: https://www.wjgnet.com/1007-9327/full/v21/i2/511.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.511
Nonalcoholic fatty liver disease (NAFLD) is a clinical syndrome characterized by hepatic steatosis and fat deposition in hepatocytes in the absence of significant alcohol use. The incidence of NAFLD in the general Chinese population has almost doubled over the last 10-15 years and is highly prevalent in obese populations[1]. Obesity-associated insulin resistance is regarded as a factor that critically contributes to the development of NAFLD[2]. Insulin resistance resulting in a hyperinsulinemic state increases de novo lipogenesis, which further exacerbates hepatic lipid deposition and boosts the development of the disease.
miRNAs are endogenously expressed RNAs consisting of 20-24 nucleotides that affect the expression of hundreds of genes involved in numerous biological processes, including lipid metabolism, organ development, differentiation, brain morphogenesis, and apoptosis.
miRNAs are potent intracellular post-transcriptional regulators and are also selectively secreted into the circulation in a cell-specific fashion. miRNAs are now known to be stably expressed in serum[3], blood[4,5] and plasma[6]. Moreover, the unique expression patterns of these circulating miRNAs are related to specific human diseases[7]. miRNA-103 (miR-103) regulates insulin sensitivity and glucose homeostasis and is highly expressed in the liver of patients with NAFLD. Furthermore, there is a positive correlation between the patient’s homeostatic model assessment (HOMA) index and miR-103 expression levels[8].
The clinical spectrum of NAFLD varies between steatosis with a benign clinical course and cirrhosis with serious complications, including hepatocellular carcinoma and liver failure, therefore, it is important to identify the molecular mechanisms and therapeutic targets of NAFLD. A “two-hit” mechanism has been proposed, however, the underlying molecular mechanism is not fully understood. In this study, we aimed to determine the levels of miR-103 expressed in the serum of patients with NAFLD to explore the associations between miR-103 and insulin resistance and NAFLD in order to identify new molecular therapeutic targets for these disorders.
This study enrolled a cohort of 50 patients with NAFLD who were treated at the Department of Endocrinology of the Second Affiliated Hospital of Medical College of Qingdao University, China from November 2011 to April 2013. Thirty age-matched healthy subjects were selected as controls. Blood pressure and electrocardiogram findings were normal in all patients. Patients with NAFLD were newly diagnosed and had not received any treatment. The diagnosis of NAFLD was based on the presence of an ultrasonographic pattern consistent with ‘‘bright liver’’ (brightness and posterior attenuation) with stronger echoes in the hepatic parenchyma than in the renal parenchyma, vessel blurring, and narrowing of the lumen of the hepatic veins in the absence of findings suggestive of other chronic liver disease. All cases of fatty liver were in accordance with the Chinese diagnostic criteria for NAFLD (alcohol consumption < 40 g per week and without consideration of alteration in liver enzymes). Body mass index (BMI) was calculated based on the following formula: BMI = weight/height2. The study was approved by the Ethics Committee of the Second Affiliated Hospital of Medical College of Qingdao University.
Fasting plasma glucose (FPG), fasting insulin (Fins), triglyceride (TG), and miR-103 levels were measured. Blood samples were obtained after an 8-h fasting period. Fins was measured by radioimmunoassay (RuiQi Biotechnology Corporation, Shanghai, China) with a sensitivity of 2 mU/L (normal range 0.5-25 mU/L). The insulin resistance index [homeostasis model assessment-insulin resistance (HOMA-IR)] was calculated using the HOMA model: (fasting insulin × fasting glucose)/22.5[9]. The expression of miR-103 was detected using real-time PCR.
Total RNA was extracted from normal controls and patients with NAFLD (Biological Technology Co. Ltd., Chengdu, China). miRNA microarray analyses were carried out by Biological Technology using an ABI3730 Sequencer (Applied Biosystems, United States).
All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, United States). Normally distributed data were expressed as mean ± SD. The t test was used to compare groups in the study. Pearson correlation analysis and stepwise regression analysis were used for simple correlation and multivariate analysis, respectively. P < 0.05 was considered statistically significant.
A total of 80 cases were included in this study. With the exception of age and gender (P > 0.05), BMI, HOMA-IR, Fins, TG, total cholesterol (TC), FPG, and alanine aminotransferase (ALT) in patients with NAFLD were higher than those in healthy controls (P < 0.05) (Table 1).
| NAFLD group (n = 50) | Normal control group (n = 30) | |
| Age (yr) | 50 ± 6.70 | 50 ± 6.81 |
| Gender (male/female) | 28/22 | 16/14 |
| BMI (kg/m2) | 28.70 ± 3.12b | 22.80 ± 3.07 |
| ln (HOMA-IR) | 1.72 ± 0.35b | 0.38 ± 0.31 |
| Fins (IU/mL) | 16.3 ± 3.06b | 8.55 ± 3.21 |
| TG (mmol/L) | 2.67 ± 1.23b | 1.58 ± 1.19 |
| TC (mmol/L) | 5.01 ± 0.89a | 4.61 ± 0.97 |
| FPG (mmol/L) | 5.95 ± 0.93b | 4.85 ± 0.87 |
| ALT (U/L) | 38.10 ± 19.80b | 26.30 ± 20.14 |
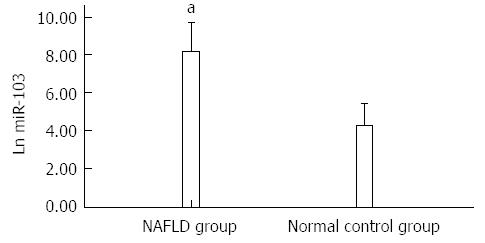
The levels of miR-103 expressed in the serum of NAFLD patients were higher than those in healthy controls (8.18 ± 0.73 vs 4.23 ± 0.81, P < 0.05) (Figure 1).
Positive correlations were observed between miR-103 and HOMA-IR (r = 0.881), BMI (r = 0.878) and TG (r = 0.774) (Figure 2).
HOMA-IR was a dependent variable and miR-103, TG and BMI were independent variables. Multivariate linear regression analyses showed that miR-103, TG and BMI were all independent factors for HOMA-IR (β = 0.438/0.657/0.251, P = 0.000/0.007/0.001) (Table 2).
| Independent variables | Independent variables | SE standard regression | t value | P value | |
| BMI | 0.251 | 0.072 | 0.431 | 3.510 | 0.001 |
| miR-103 | 0.438 | 0.113 | 0.302 | 3.890 | 0.000 |
| TG | 0.657 | 0.236 | 0.258 | 2.779 | 0.007 |
NAFLD was a dependent variable and miR-103, TG, BMI and HOMA-IR were independent variables. Binary logistic regression analysis showed that miR-103, TG, BMI and HOMA-IR were all risk factors for NAFLD (OR = 2.411/16.196/1.574/19.11, P = 0.009/0.022/0.01/0.014) (Table 3).
| Variables | P value | OR | 95%CI |
| miR-103 | 0.009 | 2.411 | 1.25-4.652 |
| TG | 0.022 | 16.196 | 1.507-174.013 |
| BMI | 0.010 | 1.574 | 1.117-20.219 |
| HOMA-IR | 0.014 | 19.11 | 1.808-202.001 |
In this study, we found that HOMA-IR, Fins, TG, and FPG levels in patients with NAFLD were higher than those in healthy controls. These results suggest that these higher levels exist in patients with insulin resistance, and lipid and glucose abnormalities. To date, despite significant efforts, the accurate pathogenesis of NAFLD is not fully understood.
NAFLD, the prevalence of which is increasing in obesity, is one of the most frequent causes of chronic liver diseases and is characterized by the accumulation of lipids in hepatic cells. It is closely associated with hypertriglyceridemia, insulin resistance and intestinal microbiota changes[10,11]. More specifically, the input of lipid exceeds the output of lipid from the liver, which induces storage of lipid in the liver contributing to the development of hepatic steatosis. According to the two-hit hypothesis, insulin resistance results in increased intrahepatic triglyceride accumulation and this is the first hit, followed by the second step. The latter likely involves cytochrome P450 activation, oxidative stress, increased inflammatory cytokine production, lipid peroxidation, activation of hepatic stellate cells, and apoptosis. França et al[12] found that hypertriglyceridemia and liver steatosis were associated with increased microsomal triglyceride transfer protein expression. Another study reported that phospholipid ω-3 polyunsaturated fatty acids may play an important role in the development of NAFLD[13]. Therefore, many of the mechanisms underlying this association are still unclear.
Insulin resistance, described as the inability of insulin to stimulate glucose uptake, is a risk factor for the development of NAFLD[14]. Insulin resistance results in a reduction in lipolysis inhibition by insulin, which leads to fatty acid accumulation contributing to altered mitochondrial function, increased lipid intermediates and hepatic steatosis[15]. Insulin activates sterol regulatory element-binding protein (SREBP)1c, a master regulatory transcription factor in lipid synthesis, through stimulation of the mammalian target of rapamycin complex 1, which leads to increased lipogenesis[16]. Therefore, insulin resistance characterized by a hyperinsulinemic state as observed in patients with NAFLD increases de novo lipogenesis, which further exacerbates hepatic lipid deposition and accelerates development of the disease.
Recent studies have indicated that miRNAs are involved in the development of NAFLD, and serum levels of miRNAs are correlated with the severity of liver steatosis[17,18] and may represent novel, noninvasive biomarkers of diagnosis and histological disease severity in patients with NAFLD[19,20]. Hoekstra et al[21] reported that fatty liver development in low-density lipoprotein receptor knockout mice was associated with a significant change in the hepatocyte miRNA profile, a fivefold decrease in miR-302a expression was reported, which predisposed the liver to insulin resistance.
miR-103 results in insulin resistance. Trajkovski et al[8] reported that silencing of miR-103 led to improved insulin resistance. In contrast, gain of miR-103 function in either liver or fat was sufficient to induce insulin resistance. Further studies confirmed that high expression of miR-103 led to insulin resistance by downregulating caveolin-1, which is the direct target gene of miR-103 and a critical regulator of the insulin receptor. In addition, silencing of miR-103 decreased total fat by reducing adipocyte size. Furthermore, adiponectin levels were increased in anti-miR-103-injected ob/ob mice. Smaller adipocytes were associated with increased insulin sensitivity in human and rodent models[22], and adiponectin levels were positively correlated with insulin sensitivity[23]. In our study, we also found that miR-103 was positively correlated with HOMA-IR and was an independent factor in overweight or obese patients with NAFLD. These data indicate that miR-103 is indirectly involved in the development of NAFLD due to insulin resistance.
Increased hepatic expression of miR-103 is directly involved in the development of NAFLD. NAFLD is highly associated with obesity and insulin resistance and is accompanied by hypertriglyceridemia, histologically characterized by hepatic TG accumulation of > 5%, resulting in steatosis. Xie et al[24] reported that miR-103 accelerates adipogenesis when expressed ectopically. miR-103 was increased in the liver of patients with NAFLD[8]. Our results showed that NAFLD patients had higher levels of miR-103 expression in serum compared with normal controls, and miR-103 was positively correlated with TG. Taken together, these results indicate that high expression of miR-103 may be directly involved in the development of NAFLD by increasing adipogenesis in hepatocytes leading to ectopic lipid deposition, thus contributing to hepatic steatosis.
miR-103 links insulin resistance and NAFLD. It has been reported that BMI and TG are the main factors related to the severity of NAFLD[25] and TG is regarded as an independent parameter associated with NAFLD[26]. Our results showed that miR-103 was positively correlated with HOMA-IR, TG and BMI, respectively, and miR-103, TG and BMI were all independent factors associated with HOMA-IR. MiR-103, TG, BMI and HOMA-IR were all risk factors for NAFLD. Therefore, miR-103 may be a potential molecular link between insulin resistance and NAFLD.
In conclusion, our results indicated that high expression of miR-103 directly increases adipogenesis in hepatocytes and indirectly results in hypertriglyceridemia due to insulin resistance, thus contributing to the development of NAFLD. Therefore, miR-103 is involved in insulin resistance and NAFLD, and may be regarded as a potential molecular link between them and a therapeutic target of these disorders. However, miR-103 as the bridge between insulin resistance and NAFLD requires further evidence. As our study included a small sample size and no liver biopsies were obtained, the above results require to be confirmed in a larger study which includes liver biopsies.
The pathogenesis of nonalcoholic fatty liver disease (NAFLD) remains obscure. Insulin resistance activates the development of NAFLD, however, the molecular mechanisms involved are not fully understood. miRNAs are involved in the development of NAFLD, and are known to be stably expressed in serum, blood and plasma. Moreover, the unique expression patterns of these circulating miRNAs are correlated with specific human diseases. miRNA-103 (miR-103) regulates insulin and glucose homeostasis and is highly expressed in the liver of patients with NAFLD.
Insulin resistance activates the development of NAFLD, and miR-103 regulates insulin sensitivity. miRNAs are involved in the development of NAFLD, and serum levels of miRNAs are correlated with the severity of liver steatosis and may represent novel, noninvasive biomarkers of diagnosis and histological disease severity in patients with NAFLD.
Previous studies have shown that miR-103 regulates insulin sensitivity in obese mice and is increased in the liver of patients with NAFLD. In this study, the authors determined fasting plasma glucose, fasting insulin, triglyceride (TG) and expressed miR-103 levels in the serum of patients with NAFLD. The levels of miR-103 expressed in serum were higher in patients with NAFLD than in controls. miR-103 was positively correlated with homeostasis model assessment-insulin resistance (HOMA-IR), TG and body mass index (BMI), respectively. miR-103 was an independent factor of HOMA-IR and a risk factor for NAFLD. Therefore, miR-103 is involved in insulin resistance and NAFLD and may be regarded as the link between them and a therapeutic target in both disorders.
The study results suggest that high expression of miR-103 directly increases adipogenesis in hepatocytes and indirectly results in hypertriglyceridemia due to insulin resistance, thus contributing to the development of NAFLD. miR-103 is involved in insulin resistance and NAFLD, and may be regarded as a potential molecular link between them and a therapeutic target in both disorders.
Insulin resistance is a physiological condition in which cells fail to respond to the normal actions of the hormone insulin. The body produces insulin, but the cells in the body become resistant to insulin and are unable to use it effectively, leading to hyperglycemia. Pancreatic β cells subsequently increase their production of insulin, further contributing to hyperinsulinemia, which is involved in the development of type 2 diabetes, hypertension, NAFLD and cancer. miR-103 belongs to the family of miRNAs, which are noncoding, highly conserved regulatory RNAs, which help to regulate gene expression at the post-transcription level. miR-103 is involved in the development of insulin resistance, cancer, and other diseases.
This was an interesting study showing the relationship between miR-103, insulin resistance and NAFLD and an interesting paper reporting novel findings regarding the role of miR-103 in the pathogenesis of NAFLD, but miR-103 as the bridge of insulin resistance and NAFLD, may need more evidence.
P- Reviewer: He JY, Tziomalos K, Wong GLH S- Editor: Qi Y L- Editor: Kerr C E- Editor: Liu XM
| 1. | Fan JG. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol. 2013;28 Suppl 1:11-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 2. | Novakovic T, Mekic M, Smilic L, Smilic T, Inić-Kostic B, Jovicevic L, Mirkovic Z, Milinic S. Anthropometric and biochemical characteristics of patients with nonalcoholic fatty liver diagnosed by non-invasive diagnostic methods. Med Arch. 2014;68:22-26. [PubMed] [Cited in This Article: ] |
| 3. | Wang F, Zheng Z, Guo J, Ding X. Correlation and quantitation of microRNA aberrant expression in tissues and sera from patients with breast tumor. Gynecol Oncol. 2010;119:586-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 4. | Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Newell J, Kerin MJ. Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann Surg. 2010;251:499-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in F6Publishing: 539] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 5. | Waters PS, McDermott AM, Wall D, Heneghan HM, Miller N, Newell J, Kerin MJ, Dwyer RM. Relationship between circulating and tissue microRNAs in a murine model of breast cancer. PLoS One. 2012;7:e50459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Zhao H, Shen J, Medico L, Wang D, Ambrosone CB, Liu S. A pilot study of circulating miRNAs as potential biomarkers of early stage breast cancer. PLoS One. 2010;5:e13735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 319] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 7. | Cookson VJ, Bentley MA, Hogan BV, Horgan K, Hayward BE, Hazelwood LD, Hughes TA. Circulating microRNA profiles reflect the presence of breast tumours but not the profiles of microRNAs within the tumours. Cell Oncol (Dordr). 2012;35:301-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, Heim MH, Stoffel M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474:649-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 707] [Cited by in F6Publishing: 751] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 9. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [PubMed] [Cited in This Article: ] |
| 10. | Takaki A, Kawai D, Yamamoto K. Molecular mechanisms and new treatment strategies for non-alcoholic steatohepatitis (NASH). Int J Mol Sci. 2014;15:7352-7379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Park JS, Seo JH, Youn HS. Gut microbiota and clinical disease: obesity and nonalcoholic Fatty liver disease. Pediatr Gastroenterol Hepatol Nutr. 2013;16:22-27. [PubMed] [Cited in This Article: ] |
| 12. | França LM, Freitas LN, Chagas VT, Coêlho CF, Barroso WA, Costa GC, Silva LA, Debbas V, Laurindo FR, Paes AM. Mechanisms underlying hypertriglyceridemia in rats with monosodium L-glutamate-induced obesity: evidence of XBP-1/PDI/MTP axis activation. Biochem Biophys Res Commun. 2014;443:725-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Lou DJ, Zhu QQ, Si XW, Guan LL, You QY, Yu ZM, Zhang AZ, Li D. Serum phospholipid omega-3 polyunsaturated fatty acids and insulin resistance in type 2 diabetes mellitus and non-alcoholic fatty liver disease. J Diabetes Complications. 2014;28:711-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Bruno Ade S, Rodrigues MH, Alvares MC, Nahas-Neto J, Nahas EA. Non-alcoholic fatty liver disease and its associated risk factors in Brazilian postmenopausal women. Climacteric. 2014;17:465-471. [PubMed] [Cited in This Article: ] |
| 15. | Geer EB, Islam J, Buettner C. Mechanisms of glucocorticoid-induced insulin resistance: focus on adipose tissue function and lipid metabolism. Endocrinol Metab Clin North Am. 2014;43:75-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 224] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 16. | Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, Lee CH. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14:21-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 458] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 17. | Miyaaki H, Ichikawa T, Kamo Y, Taura N, Honda T, Shibata H, Milazzo M, Fornari F, Gramantieri L, Bolondi L. Significance of serum and hepatic microRNA-122 levels in patients with non-alcoholic fatty liver disease. Liver Int. 2014;34:e302-e307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 110] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 18. | Yamada H, Suzuki K, Ichino N, Ando Y, Sawada A, Osakabe K, Sugimoto K, Ohashi K, Teradaira R, Inoue T. Associations between circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and non-alcoholic fatty liver. Clin Chim Acta. 2013;424:99-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 234] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 19. | Zhang Y, Cheng X, Lu Z, Wang J, Chen H, Fan W, Gao X, Lu D. Upregulation of miR-15b in NAFLD models and in the serum of patients with fatty liver disease. Diabetes Res Clin Pract. 2013;99:327-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS One. 2011;6:e23937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 423] [Cited by in F6Publishing: 436] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 21. | Hoekstra M, van der Sluis RJ, Kuiper J, Van Berkel TJ. Nonalcoholic fatty liver disease is associated with an altered hepatocyte microRNA profile in LDL receptor knockout mice. J Nutr Biochem. 2012;23:622-628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94:206-218. [PubMed] [Cited in This Article: ] |
| 23. | Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941-946. [PubMed] [Cited in This Article: ] |
| 24. | Xie H, Lim B, Lodish HF. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes. 2009;58:1050-1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 455] [Cited by in F6Publishing: 443] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 25. | Abangah G, Yousefi A, Asadollahi R, Veisani Y, Rahimifar P, Alizadeh S. Correlation of Body Mass Index and Serum Parameters With Ultrasonographic Grade of Fatty Change in Non-alcoholic Fatty Liver Disease. Iran Red Crescent Med J. 2014;16:e12669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Jiang Y, Zeng J, Chen B. Hemoglobin combined with triglyceride and ferritin in predicting non-alcoholic fatty liver. J Gastroenterol Hepatol. 2014;29:1508-1514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |