Published online May 7, 2015. doi: 10.3748/wjg.v21.i17.5352
Peer-review started: December 19, 2014
First decision: January 8, 2015
Revised: January 27, 2015
Accepted: March 12, 2015
Article in press: March 12, 2015
Published online: May 7, 2015
Processing time: 145 Days and 20.7 Hours
AIM: To investigate the safety and efficacy of adding bevacizumab to first-line chemotherapy in metastatic colorectal cancer patients with peritoneal disease.
METHODS: We compared rates of gastrointestinal perforation in patients with metastatic colorectal cancer and peritoneal disease receiving first-line chemotherapy with and without bevacizumab in three distinct cohorts: (1) the AGITG MAX trial (Phase III randomised clinical trial comparing capecitabine vs capecitabine and bevacizumab vs capecitabine, bevacizumab and mitomycinC); (2) the prospective Treatment of Recurrent and Advanced Colorectal Cancer (TRACC) registry (any first-line regimen ± bevacizumab); and (3) two cancer centres in New South Wales, Australia [Macarthur Cancer Therapy Centre and Liverpool Cancer Therapy Centre (NSWCC) from January 2005 to Decenber 2012, (any first-line regimen ± bevacizumab). For the AGITG MAX trial capecitabine was compared to the other two arms (capecitabine/bevacizumab and capecitabine/bevacizumab/mitomycinC). In the AGITG MAX trial and the TRACC registry rates of gastrointestinal perforation were also collected in patients who did not have peritoneal metastases. Secondary endpoints included progression-free survival, chemotherapy duration, and overall survival. Time-to-event outcomes were estimated using the Kaplan-Meier method and compared using the log-rank test.
RESULTS: Eighty-four MAX, 179 TRACC and 69 NSWCC patients had peritoneal disease. There were no gastrointestinal perforations recorded in either the MAX subgroup or the NSWCC cohorts. Of the patients without peritoneal disease in the MAX trial, 4/300 (1.3%) in the bevacizumab arms had gastrointestinal perforations compared to 1/123 (0.8%) in the capecitabine alone arm. In the TRACC registry 3/126 (2.4%) patients who had received bevacizumab had a gastrointestinal perforation compared to 1/53 (1.9%) in the chemotherapy alone arm. In a further analysis of patients without peritoneal metastases in the TRACC registry, the rate of gastrointestinal perforations was 9/369 (2.4%) in the chemotherapy/bevacizumab group and 5/177 (2.8%) in the chemotherapy alone group. The addition of bevacizumab to chemotherapy was associated with improved progression-free survival in all three cohorts: MAX 6.9 m vs 4.9 m, HR = 0.64 (95%CI: 0.42-1.02); P = 0.063; TRACC 9.1 m vs 5.5 m, HR = 0.61 (95%CI: 0.37-0.86); P = 0.009; NSWCC 8.7 m vs 6.8 m, HR = 0.75 (95%CI: 0.43-1.32); P = 0.32. Chemotherapy duration was similar across the groups.
CONCLUSION: Patients with peritoneal disease do not appear to have an increased risk of gastrointestinal perforations when receiving first-line therapy with bevacizumab compared to systemic therapy alone.
Core tip: This report is an analysis of three prospective studies including a randomized clinical trial. We analysed the rates of gastrointestinal perforation in patients with metastatic colorectal cancer and peritoneal disease receiving bevacizumab and systemic therapy. Previous reports had raised concerns regarding the risk of gastrointestinal perforation in this population. Our reports suggest that the absolute risk is not elevated and in addition clinicians appear to be confident in using bevacizumab in patients with colorectal cancer and peritoneal disease. We recommend that the presence of peritoneal disease is not a contraindication to the use of bevacizumab and systemic therapy.
- Citation: Roohullah A, Wong HL, Sjoquist KM, Gibbs P, Field K, Tran B, Shapiro J, Mckendrick J, Yip D, Nott L, Gebski V, Ng W, Chua W, Price T, Tebbutt N, Chantrill L. Gastrointestinal perforation in metastatic colorectal cancer patients with peritoneal metastases receiving bevacizumab. World J Gastroenterol 2015; 21(17): 5352-5358
- URL: https://www.wjgnet.com/1007-9327/full/v21/i17/5352.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i17.5352
Peritoneal spread from colorectal cancer occurs in 10%-15% of patients with metastatic colorectal cancer (mCRC)[1]. These patients typically have a high disease burden with increased morbidity and a worse prognosis than patients without peritoneal disease[2-4].
Gastrointestinal perforation is a well-documented side effect of bevacizumab, a humanized monoclonal antibody and occurs at a rate of 1%-2%. In the BRITE registry[5], 37 of 1953 metastatic colorectal patients had a gastrointestinal perforation. On multivariate analysis, risk factors associated with gastrointestinal perforation included intact primary tumour and prior adjuvant radiotherapy. The risk of gastrointestinal perforation may be enhanced in the presence of chemotherapy related gastrointestinal toxicity or by bevacizumab induced changes in tumour vasculature. A meta-analysis found gastrointestinal perforation occurs with the use of bevacizumab in other tumour types with the highest relative risk seen with intra-abdominal cancers including colorectal, ovarian, pancreatic and renal cell cancers compared to breast, lung and glioblastoma[6].
The rates of gastrointestinal perforation in phase III trials have been reported in ovarian cancer which like colorectal cancer has a predilection for peritoneal spread. In the OCEANS trial[7] and the AURELIA trial[8] the rate of gastrointestinal perforation was 0.8% and 2.2% respectively, in patients receiving bevacizumab and chemotherapy. In a univariate analysis of patients receiving bevacizumab in the GOG 0218 trial[9]-inflammatory bowel disease, small bowel surgery or large bowel surgery at time of primary resection was associated with an increased risk of gastrointestinal perforation. The increased risk of gastrointestinal perforation in patients with intra-abdominal tumours, bowel surgery or bowel obstruction have led some investigators to urge caution in using bevacizumab in patients with peritoneal metastases[6,10,11].
Our study aimed to assess the gastrointestinal safety outcomes of bevacizumab and systemic chemotherapy in mCRC patients with peritoneal disease in three cohorts, including both clinical trial and non-trial patients. The Australasian Gastrointestinal Trials Group (AGITG) MAX trial[12] included patients with peritoneal disease and hence was an ideal study for our analysis.
Adult patients with histological or radiological evidence of colorectal peritoneal metastases were identified from three sources: the AGITG MAX study[12], the TRACC registry (Treatment of Recurrent and Advanced Colorectal Cancer) and two community cancer centres in New South Wales, Australia (NSWCC).
The MAX study was an international phase III, open label, randomised clinical trial with three arms: (1) capecitabine; (2) capecitabine and bevacizumab; and (3) capecitabine, mitomycinC and bevacizumab. Data was collected prospectively in detailed case report forms and patients were recruited between July 2005 and June 2007. Inclusion and exclusion criteria have been previously published[12].
The TRACC registry is a multi-site prospective clinical registry established by BioGrid Australia in 2009 and supported by Roche Australia Pty Ltd. A consensus dataset, developed by a panel of clinicians, is used to capture comprehensive clinical, treatment and outcome data for consecutive patients with mCRC. Data are entered directly by clinicians into an electronic database[13]. Patient enrolment is ongoing at 24 sites across Australia. For this analysis, data was available from 16 sites.
The NSWCC cohort was identified from two cancer centres in New South Wales - Macarthur Cancer Therapy Centre and Liverpool Cancer Therapy Centre. A search of the electronic medical records was performed to identify patients who had started any first line chemotherapy between January 2005 and December 2012.
Baseline information was collected including site of tumour, distribution of metastatic disease, height, weight, age, gender and performance status. Treatment information was collected: chemotherapy regimen, number of completed chemotherapy cycles (≥ 90% of each prescribed dose), abdominal adverse events of grade ≥ 2 occurring during first line treatment (abdominal pain, ascites, fistula, gastrointestinal perforations, gastrointestinal obstruction and tumour related haemorrhage) and CT assessments. Progression was determined by radiological or clinical progression using RECIST 1.0 Criteria.
The primary endpoint of this analysis of all three cohorts was the rate of gastrointestinal perforation. Secondary endpoints included progression-free survival (PFS), duration of chemotherapy and overall survival (OS). PFS was defined as the time from baseline to progression or death. For all cohorts bevacizumab and chemotherapy was compared to chemotherapy alone.
Toxicity and treatment duration were analysed by treatment received. Survival analyses were by intention-to-treat in the MAX trial and by treatment received in the other cohorts. Survival endpoints were analysed by Kaplan-Meier curves and compared using unadjusted log rank tests. The statistical methods of this study were reviewed by the biostatistician-Prof Val Gebski of the NHMRC Clinical Trials Centre.
In the MAX trial there were 84 patients with peritoneal disease with a median follow up of 30.8 mo. Thirty-three received capecitabine alone and 51 received chemotherapy/bevacizumab. There were no significant clinicopathological differences between the two bevacizumab arms and the capecitabine arm.
In the TRACC registry, 179 patients with peritoneal disease were identified with a median follow up of 26.3 mo. Fifty-three patients had chemotherapy alone and 126 patients had chemotherapy/bevacizumab. There were relevant differences in clinicopathological factors such as higher age, worse performance status, less use of doublet chemotherapy and more non- resected primaries in the chemotherapy alone arm (Table 1).
| MAX | TRACC | NSWCC cohort | ||||
| July 2005 to June 2007 | July 2009 to June 2014 | Jan 2005 to Dec 2012 | ||||
| Chemo alone | Chemo + Bev | Chemo alone | Chemo + Bev | Chemo alone | Chemo + Bev | |
| n | 33 | 51 | 53 | 126 | 52 | 17 |
| Age (yr) | ||||||
| Median | 70 | 67 | 72 | 64 | 66 | 60 |
| Range | 47-84 | 39-83 | 31-91 | 30-87 | 38-83 | 21-79 |
| Sex | ||||||
| Male | 15 | 30 | 22 | 71 | 35 | 11 |
| Female | 18 | 21 | 31 | 55 | 17 | 6 |
| ECOG | ||||||
| PS 0 | 14 | 29 | 8 | 45 | 35 | 13 |
| PS 1 | 17 | 15 | 28 | 61 | 12 | 3 |
| PS 2 | 2 | 7 | 14 | 17 | 5 | 1 |
| PS 3 | NA | NA | 3 | 3 | NA | NA |
| CCI | NA | NA | ||||
| 0 | 33 | 89 | ||||
| 1-2 | 14 | 33 | ||||
| ≥ 3 | 6 | 4 | ||||
| Primary site | ||||||
| Colon | 29 | 44 | 48 | 107 | 47 | 15 |
| Rectum | 4 | 7 | 5 | 14 | 5 | 2 |
| Occult | 5 | |||||
| Prior adjuvant Rx | ||||||
| Yes | 5 | 11 | 10 | 34 | 11 | 4 |
| No | 28 | 40 | 43 | 92 | 41 | 13 |
| Primary resected | ||||||
| Yes | 28 | 39 | 24 | 80 | 34 | 16 |
| No | 5 | 12 | 29 | 46 | 18 | 1 |
| No. of met sites | NA | NA | ||||
| 1 | 15 | 30 | ||||
| 2 | 17 | 45 | ||||
| ≥ 3 | 21 | 51 | ||||
| Chemo regimen | NA | NA | ||||
| Single-agent FP | 19 | 12 | ||||
| Oxaliplatin-based doublet | 25 | 87 | ||||
| Irinotecan-based doublet | 6 | 18 | ||||
| Single-agent Irinotecan | 0 | 3 | ||||
| Other | 3 | 6 | ||||
In the NSWCC patients there were 69 patients identified, 52 received chemotherapy alone and 17 received chemotherapy/bevacizumab. In the NSWCC patients who received chemotherapy alone, 23/52 (44.2%) had a documented relative contraindication to bevacizumab. Of those without a contraindication 16/29 (55.2%) were treated with chemotherapy alone prior to 2009 when bevacizumab was not publicly funded. Table 1 summarises the baseline characteristics.
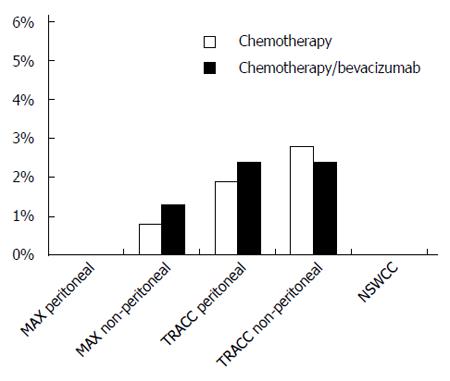
There were no gastrointestinal perforations recorded in either the MAX subgroup or the NSWCC cohorts. Of the patients without peritoneal disease in the MAX trial, 4/300 (1.3%) in the bevacizumab arms had gastrointestinal perforations compared to 1/123 (0.8%) in the capecitabine alone arm. In the TRACC registry 3/126 (2.4%) patients who had received bevacizumab had a gastrointestinal perforation compared to 1/53 (1.9%) in the chemotherapy alone arm. In a further analysis of patients without peritoneal metastases in the TRACC registry, the rate of gastrointestinal perforations was 9/369 (2.4%) in the chemotherapy/bevacizumab group and 5/177 (2.8%) in the chemotherapy alone group (Figure 1).
In the MAX trial 9/33 (27.3%) patients in the capecitabine arm had grade ≥ 2 diarrhoea compared to 21/51 (41.2%) in the two bevacizumab arms. There was one patient with tumour associated haemorrhage event in the capecitabine arm and one patient in the bevacizumab arms.
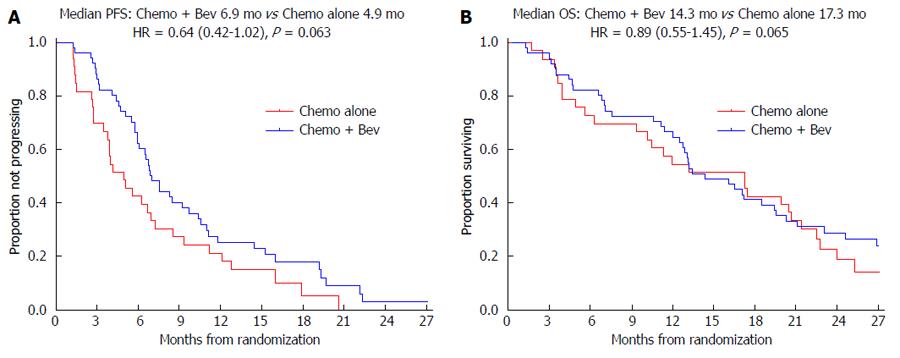
In the MAX trial median PFS was 6.9 mo in the two bevacizumab arms compared to 4.9 mo in the capecitabine alone arm with a HR = 0.64, (95%CI: 0.42-1.02); P = 0.063 (Figure 2A). In the TRACC registry the median PFS was 9.1 mo in the chemotherapy/bevacizumab group compared to 5.5 mo in the chemotherapy alone group; HR = 0.61, (95%CI: 0.37-0.86); P = 0.009. In the NSWCC cohort the median PFS was 8.7 mo in the chemotherapy/bevacizumab group compared to 6.8 mo in the chemotherapy alone group; HR = 0.75, (95%CI: 0.43-1.32); P = 0.32.
In the MAX trial median OS was 14.3 mo in the two bevacizumab arms compared to 17.3 mo in the capecitabine alone arm; HR = 0.89, 95%CI: 0.55-1.45; P = 0.65 (Figure 2B). In the TRACC registry the median OS was 20.0 mo in the chemotherapy/bevacizumab group compared to 14.7 mo in the chemotherapy alone arm; HR = 0.60, 95%CI: 0.34-0.88; P = 0.013. In the NSWCC cohort the median OS was 23.4 mo in chemotherapy/bevacizumab group compared to 13.7 mo in the chemotherapy alone group, HR = 0.62, 95%CI: 0.33-1.17; P = 0.14.
In the MAX trial the median number of chemotherapy cycles in the two bevacizumab arms was 8 cycles and 6 cycles in the capecitabine arm. In the NSWCC cohort the median duration of chemotherapy in the chemotherapy/bevacizumab group was 21 wk compared to 14 wk chemotherapy alone group.
Despite the advances in the treatment of metastatic colorectal cancer, the prognosis of patients with peritoneal disease remains poor. This analysis suggests that the addition of bevacizumab to chemotherapy does not increase the rate of gastrointestinal perforations in patients with metastatic colorectal cancer and peritoneal metastases. Specifically, no gastrointestinal perforations were seen in the patients with peritoneal disease in the MAX study and the NSWCC cohort, and in the TRACC registry bevacizumab use was not associated with an increased rate of gastrointestinal perforation. The addition of bevacizumab to standard first-line chemotherapy is associated with longer PFS in patients with peritoneal disease treated in the trial and non-trial settings. There was a consistent PFS benefit seen across studies, with the hazard ratios ranging from 0.61 to 0.75, with statistical significance only seen in the TRACC registry. However, the AGITG MAX clinical trial was not powered to detect statistically significant improvements in PFS within the peritoneal subgroup. Significant differences in prognostic variables, as well as selection bias may have led to the significant survival differences associated with bevacizumab use in the TRACC registry and the NSWCC cohort. Both the TRACC registry and NSWCC cohorts demonstrated a > 50% usage of bevacizumab alongside a range of chemotherapy regimens by clinicians.
Multiple risk factors have been associated with an increased risk of gastrointestinal perforations. Intra-abdominal tumours such colorectal, ovarian, pancreatic and renal cell cancers have been associated with an elevated risk of gastrointestinal perforation compared to non-abdominal cancers such as breast and lung cancers[6,11]. In the BRITE registry[5] the rate of gastrointestinal perforation in all patients with mCRC was 1.9% with an elevated risk on multivariate analysis seen in patients with an intact primary tumour and prior adjuvant radiation. Given the increased risk of gastrointestinal perforations in patients with mCRC investigators have postulated various mechanisms including predisposing GI toxicity from chemotherapy, pelvic irradiation, intact primary tumour, peritoneal metastases and bevacizumab-induced changes in tumour vasculature. To our knowledge the risk of gastrointestinal perforation in patients with peritoneal disease has been poorly described. The AGITG MAX trial is the only randomised Phase III trial comparing a bevacizumab containing arm to a non-bevacizumab containing arm where the outcomes of peritoneal disease patients are described. Although others have performed analyses of patients with peritoneal metastases in randomised trials, these were not comparisons of the addition of bevacizumab to chemotherapy[3,4,14]. In most cases safety analyses were not reported.
Our analysis presents outcomes of patients in a clinical trial as well as outcomes in patients treated in typical community cancer centres. Rates of gastrointestinal perforations were available from all three cohorts and indicate that bevacizumab appears to be as safe in the peritoneal disease subgroup as compared to patients without peritoneal disease. Furthermore, despite previous series suggesting an elevated risk[6,10,11], clinicians appear to be comfortable in using bevacizumab in patients with peritoneal disease as reflected in the number of patients with peritoneal disease recruited to MAX and the proportion of bevacizumab-treated patients in the TRACC registry not being impacted by the presence or absence of peritoneal disease. The weaknesses of the analysis include the small patient numbers in each cohort. In Australia, bevacizumab has been publicly funded since 2009 and thus the numbers of patients with peritoneal disease who had access to bevacizumab in the NSWCC were small. Nevertheless, this analysis represents the largest analysis of gastrointestinal perforations in patients with peritoneal disease.
There is significant discordance seen in the OS effects. The median OS in the MAX trial is longer in the capecitabine alone arm, however, the curves cross before and after the median with a HR = 0.89. Use of post-progression therapy was minimal in the MAX trial, suggesting imbalances in subsequent therapy do not explain the discordance between the impact on PFS and OS. Nonetheless, the OS findings of the subgroup of patients with peritoneal metastases are consistent with the overall MAX trial results. While a statistically significant improvement in OS was seen in the TRACC registry, this may be due to differences in prognostic factors between the bevacizumab and non-bevacizumab treated patients given the non-randomised nature of the registry, which likely account for at least some of this gain. Such an imbalance in patient prognostic factors may also have explained the inconsistency in data from the BRITE registry[15], which showed a far larger survival gain with bevacizumab beyond progression in all patients with metastatic colorectal cancer than was seen in the randomised TML trial[16]. Although the difference in survival is large in the NSWCC cohort there are known significant baseline differences between the groups and these factors may account for the difference seen.
Alternative routes of bevacizumab administration may be helpful for patients with peritoneal disease, with case reports of intraperitoneal administration of bevacizumab with significant symptomatic benefit mainly in ovarian cancer patients[17-19]. However, we are unaware of any data related to the safety and efficacy of this approach in metastatic colorectal cancer. The results of ongoing trials are eagerly awaited[20].
In conclusion, this analysis of metastatic colorectal cancer patients with peritoneal disease reveals similar findings across a clinical trial and routine practice cohorts. The combination appears to be safe to use, without an increase in rate of gastrointestinal perforations observed in any of the cohorts. From our analysis, patients with metastatic colorectal cancer to the peritoneum should not be excluded from receiving bevacizumab.
Bevacizumab is associated with gastrointestinal perforation with known risk factors such as intra-abdominal cancers, intact primary tumours and bowel obstruction. Initial use of bevacizumab in ovarian cancer was associated with a higher than expected rate of gastrointestinal perforation however rates in recent clinical trials are not elevated compared to rates in other malignancies. However, there remains a concern of gastrointestinal perforations in patients with metastatic colorectal cancer and peritoneal metastases.
There are currently no reports in the literature to our knowledge that have looked solely at metastatic colorectal cancer with peritoneal metastases and the risk of gastrointestinal perforations with the use of bevacizumab and chemotherapy. There have been reports that have looked at other risk factors for this adverse event including registry reports and clinical trial results.
The authors used three prospective sources of data to shows results in clinical trial and community settings. This allowed a higher number of patients with exposure to bevacizumab and chemotherapy and increases the confidence that the rate of gastrointestinal perforation in patients with metastatic colorectal cancer and peritoneal metastases is not elevated. The results of this study are consistent with recent clinical trial results in ovarian cancer which also has a predilection for peritoneal disease.
Clinicians should be confident using bevacizumab and chemotherapy in patients with metastatic colorectal cancer and peritoneal disease who do not have other contraindications.
Gastrointestinal perforation includes perforation of the oesophagus, stomach, small bowel or large bowel which is usually life threatening or fatal. A grade 3 perforation is defined as the presence of serious symptoms and surgical intervention is indicated.
The authors contribute their meaningful work regarding a side effect of bevacizumab. The data was extracted from trials, prospective registry and data set from cancer centres and was analysed thoroughly.
P- Reviewer: Lu XF, Tanriverdi O S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243:212-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 400] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 2. | Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JW, de Hingh IH. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer. 2011;128:2717-2725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 3. | Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, Pitot HC, Grothey A, Alberts SR, Sargent DJ. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012;30:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 427] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 4. | Klaver YL, Simkens LH, Lemmens VE, Koopman M, Teerenstra S, Bleichrodt RP, de Hingh IH, Punt CJ. Outcomes of colorectal cancer patients with peritoneal carcinomatosis treated with chemotherapy with and without targeted therapy. Eur J Surg Oncol. 2012;38:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Kabbinavar FF, Flynn PJ, Kozloff M, Ashby MA, Sing A, Barr CE, Grothey A. Gastrointestinal perforation associated with bevacizumab use in metastatic colorectal cancer: results from a large treatment observational cohort study. Eur J Cancer. 2012;48:1126-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009;10:559-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 7. | Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, Sovak MA, Yi J, Nycum LR. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 984] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 8. | Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P, Bamias A. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 1115] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 9. | Burger RA, Brady MF, Bookman MA, Monk BJ, Walker JL, Homesley HD, Fowler J, Greer BE, Boente M, Fleming GF. Risk factors for GI adverse events in a phase III randomized trial of bevacizumab in first-line therapy of advanced ovarian cancer: A Gynecologic Oncology Group Study. J Clin Oncol. 2014;32:1210-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Abu-Hejleh T, Mezhir JJ, Goodheart MJ, Halfdanarson TR. Incidence and management of gastrointestinal perforation from bevacizumab in advanced cancers. Curr Oncol Rep. 2012;14:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Qi WX, Shen Z, Tang LN, Yao Y. Bevacizumab increases the risk of gastrointestinal perforation in cancer patients: a meta-analysis with a focus on different subgroups. Eur J Clin Pharmacol. 2014;70:893-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Tebbutt NC, Wilson K, Gebski VJ, Cummins MM, Zannino D, van Hazel GA, Robinson B, Broad A, Ganju V, Ackland SP. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol. 2010;28:3191-3198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 327] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 13. | Field K, Wong HL, Shapiro J, Kosmider S, Tie J, Bae S, Yip D, McKendrick J, Nott L, Desai J. Developing a national database for metastatic colorectal cancer management: perspectives and challenges. Intern Med J. 2013;43:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Chua TC, Morris DL, Saxena A, Esquivel J, Liauw W, Doerfer J, Germer CT, Kerscher AG, Pelz JO. Influence of modern systemic therapies as adjunct to cytoreduction and perioperative intraperitoneal chemotherapy for patients with colorectal peritoneal carcinomatosis: a multicenter study. Ann Surg Oncol. 2011;18:1560-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, Kozloff M. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008;26:5326-5334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 505] [Article Influence: 29.7] [Reference Citation Analysis (1)] |
| 16. | Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 883] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 17. | Hamilton CA, Maxwell GL, Chernofsky MR, Bernstein SA, Farley JH, Rose GS. Intraperitoneal bevacizumab for the palliation of malignant ascites in refractory ovarian cancer. Gynecol Oncol. 2008;111:530-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Bellati F, Napoletano C, Ruscito I, Pastore M, Pernice M, Antonilli M, Nuti M, Benedetti Panici P. Complete remission of ovarian cancer induced intractable malignant ascites with intraperitoneal bevacizumab. Immunological observations and a literature review. Invest New Drugs. 2010;28:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | El-Shami K, Elsaid A, El-Kerm Y. Open-label safety and efficacy pilot trial of intraperitoneal bevacizumab as palliative treatment in refractory malignant ascites (Abstract only). J Clin Oncol. 2007;25:Abstract 9043. |
| 20. | Sjoquist KM, Friedlander M, Mileshkin LR, Quinn M, Goh JC, Shannon CM, Bowtell D, Plebanski M, Yip S, Carlton K. The REZOLVE phase II trial to evaluate the safety and potential palliative benefit of intraperitoneal bevacizumab in patients with symptomatic ascites due to advanced, chemotherapy-resistant ovarian cancer (Abstract only). J Clin Oncol. 2014;32:Abstract TPS5627. |