Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4255
Peer-review started: October 14, 2014
First decision: October 29, 2014
Revised: January 16, 2015
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: April 14, 2015
Processing time: 196 Days and 18.8 Hours
AIM: To investigate the prognostic value of metastatic lymph node ratio (MLNR) in extrahepatic cholangiocarcinoma (ECC) patients undergoing radical resection.
METHODS: Seventy-eight patients with ECC were enrolled. Associations between various clinicopathologic factors and prognosis were investigated by Kaplan-Meier analyses. The Cox proportional-hazards model was used for multivariate survival analysis.
RESULTS: The overall three- and five-year survival rates were 47.26% and 23.99%, respectively. MLNR of 0, 0-0.2, 0.2-0.5, and > 0.5 corresponded to five-year survival rates of 28.59%, 21.60%, 18.84%, and 10.03%, respectively. Univariate analysis showed that degree of tumor differentiation, lymph node metastasis, MLNR, tumor-node-metastasis (TNM) stage, and margin status were closely associated with postoperative survival in ECC patients (P < 0.05). Multivariate analysis showed that MLNR and TNM stage were independent prognostic factors after pancreaticoduodenectomy (HR = 2.13, 95%CI: 1.45-3.11; P < 0.01; and HR = 1.97, 95%CI: 1.17-3.31; P = 0.01, respectively). The median survival time for MLNR > 0.5, 0.2-0.5, 0-0.2, and 0 was 15 mo, 24 mo, 23 mo, and 35.5 mo, respectively. There were statistical differences in survival time between patients with different MLNR (χ2 = 15.38; P < 0.01).
CONCLUSION: MLNR is an independent prognostic factor for ECC patients after radical resection and is useful for predicting postoperative survival.
Core tip: This study aims to investigate the prognostic significance of metastatic lymph node ratio in extrahepatic cholangiocarcinoma patients undergoing radical resection. Using univariate and multivariate analysis, we found that metastatic lymph node ratio was an independent prognostic factor for these patients after radical resection and is useful for predicting postoperative survival.
- Citation: Zhang JW, Chu YM, Lan ZM, Tang XL, Chen YT, Wang CF, Che X. Correlation between metastatic lymph node ratio and prognosis in patients with extrahepatic cholangiocarcinoma. World J Gastroenterol 2015; 21(14): 4255-4260
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4255.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4255
Cholangiocarcinoma (CCA) is a malignant tumor that originates from the intra- and extrahepatic biliary epithelium, and it accounts for approximately 3% of all gastrointestinal malignancies[1]. Patients with liver fluke infestation, chronic viral hepatitis, choledochal cysts, and primary sclerosing cholangitis can develop CCA[2]. Intrahepatic CCA arises within the hepatic parenchyma, and most often presents as a mass lesion without major bile duct obstruction or jaundice[3]. Extrahepatic cholangiocarcinoma (ECC) is defined as common bile duct CCA, which accounts for 20%-40% of CCA cases[4]. At present, surgical resection remains the only treatment choice for ECC patients. However, the curative rate of ECC has been low for patients in advanced stages[5]. Even with complete resection of the tumors, most patients are subject to local recurrence or distant metastasis[6]. According to the staging of extrahepatic bile duct cancer, the number of metastatic lymph nodes is a key parameter for tumor staging and prognosis prediction. Lymph node metastasis is a prognostic factor for survival of ECC patients after curative resection[7], and those with peripheral lymph node metastases had notably poorer prognosis.
Metastatic lymph node ratio (MLNR), the ratio of the number of metastatic lymph nodes to the number of lymph nodes removed, is regarded as an important prognostic factor for various tumors[8-14]. However, there are few studies examining the association between MLNR and prognosis in ECC patients. In this study, we analyzed multiple clinicopathologic factors in ECC patients, and investigated the potential association between lymph node metastasis and prognosis. We aimed to find reliable indicators for predicting the prognosis of ECC patients following radical resection.
A total of 128 ECC patients were recruited from the Cancer Hospital of Chinese Academy of Medical Sciences between January 1999 and January 2012. The recruited patients needed to meet the following inclusion criteria: (1) complete clinical data available; (2) pathologically confirmed ECC after surgery; (3) neoadjuvant chemoradiotherapy-naïve before surgery; (4) complete follow-up record available (until January 2014); and (5) absence of liver disease or other diseases. All patients received pancreaticoduodenectomy and preoperative assessment, including a detailed history and physical, laboratory, and radiologic examinations. All patients underwent enhanced abdominal CT/magnetic resonance imaging, abdominal ultrasound, and determination of serum tumor markers.
The tumors were classified based on the tumor-node-metastasis (TNM) classification criteria of the American Joint Committee on Cancer (AJCC), 6th edition[15]. The clinicopathologic data analyzed in this study included: age, sex, duration of operation, intraoperative blood loss, tumor differentiation, tumor embolism, perineural invasion, T component of TNM stage, TNM stage, margin status, postoperative adjuvant chemotherapy, total number of dissected lymph nodes, lymph node status, and MLNR.
All patients underwent lymphadenectomy; based on the Japanese Pancreatic Society classification of pancreatic cancer, the extent of lymphadenectomy was defined as follows: around the pancreas and duodenum (stations 13 and 17), inside the hepatoduodenal ligament (station 12), around the stomach (stations 1-6), around the hepatic artery proper (station 8), and around the superior mesenteric artery (station 14).
Data on the total number of lymph nodes dissected and the number of lymph node metastases were obtained from pathologic reports. Patients were divided into four groups according to the MLNR values: patients with negative lymph nodes (MLNR = 0), and patients with positive lymph nodes (0 < MLNR < 0.2, 0.2 < MLNR < 0.5 and 0.5 < MLNR).
Follow-up was performed via telephone or mail, and all outpatient records were reviewed. The first follow-up visit was made at 6 mo after surgery. It was then continued every 6-12 mo until March 2014.
Statistical analysis was performed using the SAS v 9.2 (SAS Institute Inc., Cary, NC, United States). The life-table method was used to calculate the three- and five-year survivals. The Kaplan-Meier method was used to construct survival curves, which were compared using the log-rank test. Multivariate analysis of prognostic factors was performed using the Cox proportional-hazards model. Survival was calculated from the day of surgery to the time of death (for non-surviving patients) or to the last follow-up (until March 2014 for surviving patients or patients who dropped out). P < 0.05 was considered statistically significant.
Seventy-eight patients, including 51 men and 27 women, were included in the final analysis. Their average age was 60.2 years, ranging from 42 to 78 years. Two patients were classified as stage I, 26 as stage II, and 50 as stage III. Fifty-five patients were diagnosed with lymph node metastasis. The average number of dissected lymph nodes was 15.4 (range: 10-36). Forty-two patients were lost to follow-up. Eight patients were excluded, among who four were without complete clinical information, two were diagnosed with non-ECC, one had received adjuvant chemotherapy before operation, and one had received interventional chemotherapy before operation.
The overall three- and five-year survival rates were 47.26% and 23.99%, respectively. There were no statistically significant differences in the survival rates with regard to age, sex, duration of surgery, intraoperative blood loss, perineural invasion, tumor embolism, T stage, number of lymph node dissected, or postoperative chemotherapy. The three- and five-year survival rates of patients with peripheral lymph node metastasis (37.74% and 17.56%, respectively) were lower than those without peripheral lymph node metastasis (70.13% and 28.59%, respectively), and the differences were statistically significant (Ps < 0.05). Five-year survival rates according to MLNR were: 28.59% (MLNR = 0), 21.60% (MLNR = 0-0.2), 18.84% (MLNR = 0.2-0.5), and 10.03% (MLNR > 0.05).
Univariate analyses showed that degree of tumor differentiation, lymph node metastasis, MLNR, TNM stage, and margin status were significantly correlated with postoperative survival in ECC patients (all P < 0.05) (Table 1). Furthermore, the Cox proportional-hazard model for multivariate analysis was used to further investigate these factors, showing that MLNR and TNM stage were independent predictors of survival (Table 2).
| Clinicopathologic factors | No. of patients | Survival (%) | P valuea | |
| 3-yr | 5-yr | |||
| Total cases | 78 | 47.26 | 23.99 | |
| Age (yr) | 0.388 | |||
| ≤ 60 | 46 | 57.14 | 28.57 | |
| > 60 | 32 | 45.22 | 26.65 | |
| Sex | 0.748 | |||
| Male | 51 | 46.92 | 21.90 | |
| Female | 27 | 48.48 | 36.36 | |
| Duration of surgery (min) | 0.763 | |||
| ≤ 300 | 41 | 46.27 | 27.76 | |
| > 300 | 37 | 47.98 | 18.66 | |
| Intraoperative blood loss (mL) | 0.337 | |||
| ≤ 500 | 47 | 57.14 | 28.57 | |
| > 500 | 31 | 46.34 | 26.42 | |
| Differentiation degree | < 0.01 | |||
| Highly | 24 | 61.32 | 33.45 | |
| Moderately | 44 | 50.00 | 28.04 | |
| Poorly | 10 | 23.08 | 15.38 | |
| Perineural invasion | 0.435 | |||
| Yes | 57 | 38.72 | 14.75 | |
| No | 21 | 68.38 | 48.84 | |
| Tumor embolism | 0.183 | |||
| Yes | 3 | 33.33 | 33.33 | |
| No | 75 | 47.94 | 25.68 | |
| T stage | 0.369 | |||
| T1 | 2 | 100 | 50 | |
| T2 | 7 | 53.07 | 33.77 | |
| T3 | 24 | 46.05 | 21.98 | |
| T4 | 45 | 37.40 | 18.70 | |
| Total number of lymph node dissected | 0.179 | |||
| ≤ 15 | 30 | 45.92 | 26.53 | |
| > 15 | 48 | 39.39 | 26.26 | |
| Lymph node metastasis | 0.010 | |||
| Yes | 55 | 37.74 | 17.56 | |
| No | 23 | 70.13 | 28.59 | |
| MLNR | 0.002 | |||
| 0 | 23 | 70.13 | 28.59 | |
| 0-0.2 | 12 | 54.01 | 21.60 | |
| 0.2-0.5 | 18 | 48.34 | 18.84 | |
| > 0.5 | 25 | 33.67 | 10.03 | |
| TNM stage | 0.044 | |||
| I | 2 | 54.83 | 27.95 | |
| II | 26 | 41.67 | 21.03 | |
| III | 50 | 35.06 | 17.53 | |
| Cutting edge | 0.043 | |||
| Negative | 75 | 54.36 | 25.02 | |
| Positive | 3 | 33.33 | 10.00 | |
| Postoperative chemotherapy | 0.055 | |||
| Yes | 64 | 54.55 | 22.02 | |
| No | 14 | 46.80 | 22.40 | |
| Factors | β | SD | χ2 | P value | HR | 95%CI |
| MLNR | 0.75 | 0.19 | 15.01 | < 0.01 | 2.13 | 1.45–3.11 |
| TNM stage | 0.67 | 0.26 | 6.55 | 0.011 | 1.97 | 1.17–3.31 |
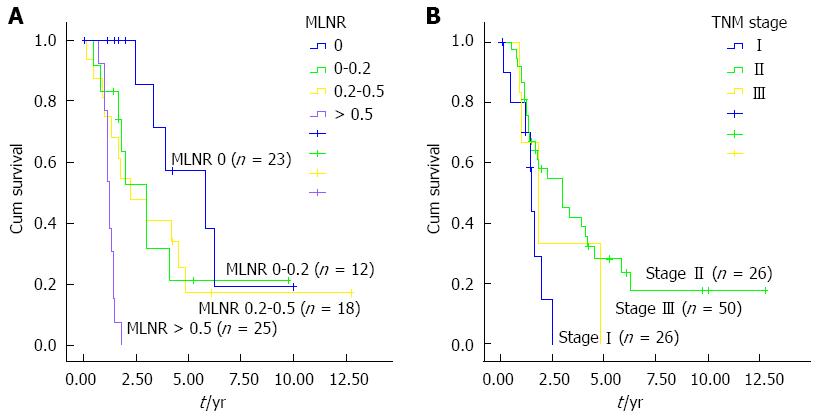
To further determine the effects of MLNR and TNM stage on prognosis of patients, survival curves were established. Median survival time for regional lymph node metastases > 0.5, 0.2-0.5, 0-0.2, and 0 were 15 mo, 24 mo, 23 mo, and 35.5 mo, respectively. The log-rank test revealed significant differences in survival time among patients with different MLNR values (χ2 = 15.376; P < 0.01) (Figure 1A). Median survival time for TNM stage I, II, and III were 15.5 mo, 24.0 mo, 23.0 mo, and 35.5 mo, respectively, with significant differences (χ2 = 15.376; P < 0.01) (Figure 1B).
Some factors have been found for the prognosis of CCA[16-21]. However, there are few studies on survival outcomes and prognostic factors of ECC patients[22]. The TNM staging system has been widely applied as a simple, convenient, and repeatable method. For ECC patients, the N component, or description of the involvement of regional lymph nodes, is based on the number and location of metastatic lymph nodes retrieved intraoperatively, and is used to predict prognosis. According to the 6th edition of the AJCC Cancer Staging Manual, lymphadenectomy should be considered in patients with more than 15 lymph nodes, which is evaluated as the N component of the TNM stage[23]. All these indicate that the N component may not accurately predict prognosis of ECC patients with fewer than 15 lymph nodes removed. However, the number of lymph nodes removed is often dictated by the knowledge and skill of the surgeon and pathologist. Therefore, MLNR is a more reliable prognostic factor than the number of metastatic lymph nodes[24], and the prognostic value of MLNR is not influenced by the scope of lymph node dissection[25-27].
In the present study, we performed univariate and multivariate analyses to investigate the role of MLNR in prognosis prediction of ECC patients, and found that MLNR is an independent prognostic factor. We also analyzed the prognostic values of other lymph node-related indicators and found that lymph node metastasis, but not the total number of lymph nodes dissected, was closely associated with prognosis. In a retrospective analysis of 93 intrahepatic CCA patients, Tamandl et al[28] verified that the total number of lymph nodes dissected was not associated with prognosis. Although no evidence has demonstrated that dissection of more lymph nodes improves the prognosis, extended lymphadenectomy can more accurately identify the status of lymph node metastasis and predict prognosis[29]. As the scope of lymphadenectomy during radical resection of ECC remains controversial, MLNR is particularly important for evaluating the prognosis of ECC patients, which can represent the number and location of metastatic lymph nodes. Furthermore, calculation of MLNR is a simple and highly repeatable method for stratification of outcomes and takes into account not only the number of dissected lymph nodes, but also biologic behavior (i.e., number of positive lymph nodes).
In the present study, MLNR was found to be an independent prognostic indicator of long-term patient survival; a higher MLNR value predicted poorer biologic behavior and prognosis. MLNR can be used in postoperative stratification of ECC patients, i.e., assessment of the appropriateness of further treatment or enrollment in future clinical trials.
In summary, MLNR is an independent prognostic factor for ECC patients who underwent pancreaticoduodenal resection. MLNR can be used as an important tool in postoperative pathologic evaluation to predict prognosis and facilitate stratification for treatment. More cases of ECC should be considered in further studies for verifying the association between MLNR and survival.
Extrahepatic cholangiocarcinoma (ECC) is a gastrointestinal malignancy with poor prognosis. Surgical resection remains the only treatment choice for ECC. However, the curative rate of ECC has been low for patients diagnosed with advanced stages. There are no effective methods for predicting postoperative survival of patients with ECC.
Metastatic lymph node ratio (MLNR), the ratio of the number of metastatic lymph nodes to the number of lymph nodes removed, is known as an important prognostic factor for various tumors.
There are few studies examining the association between MLNR and prognosis in ECC patients. The authors firstly investigated the prognostic factors for ECC in a Chinese population. A total of 128 ECC cases were collected from January 1999 to January 2012. Multivariate analysis was performed to investigate the association between clinicopathologic factors and the survival of ECC patients. The Kaplan-Meier method was used to construct survival curves and to investigate the clinicopathologic factors for the survival of ECC patients.
MLNR is an independent prognostic factor for patients with ECC after radical resection, which may be used as an index for predicting postoperative survival.
Cholangiocarcinoma is a malignant tumor that originates from the intra- and extrahepatic biliary epithelium, and accounts for ~3% of all gastrointestinal malignancies. ECC is defined as cholangiocarcinoma of the common bile duct, which accounts for 20-40% of the cases.
It is a good retrospective study in which the authors investigated the prognostic factors for patients with ECC. The results are interesting and suggest that MLNR is an independent prognostic factor for patients with distal cholangiocarcinoma after radical resection and is useful for predicting postoperative survival.
P- Reviewer: Andersson RG, Parsi MA, Pinlaor S, Plentz RR, Vegso G S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Ma S
| 1. | Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, Wiangnon S, Sripa B, Hong ST. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 334] [Article Influence: 22.3] [Reference Citation Analysis (2)] |
| 2. | Braconi C, Patel T. Cholangiocarcinoma: new insights into disease pathogenesis and biology. Infect Dis Clin North Am. 2010;24:871-84, vii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 1067] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 4. | Nishimura M, Naka S, Hanazawa K, Tani T, Fukami M, Okada S, Fujiyama Y. Cholangiocarcinoma in the distal bile duct: a probable etiologic association with choledocholithiasis. Dig Dis Sci. 2005;50:2153-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Gwak HK, Kim WC, Kim HJ, Park JH. Extrahepatic bile duct cancers: surgery alone versus surgery plus postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 2010;78:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakamura H, Nakashima A, Sueda T. Adjuvant gemcitabine plus S-1 chemotherapy improves survival after aggressive surgical resection for advanced biliary carcinoma. Ann Surg. 2009;250:950-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Kim HJ, Kim CY, Hur YH, Koh YS, Kim JC, Kim HJ, Cho CK. The prognostic factors for survival after curative resection of distal cholangiocarcinoma: perineural invasion and lymphovascular invasion. Surg Today. 2014;44:1879-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23:8706-8712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 408] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 9. | Nitti D, Marchet A, Olivieri M, Ambrosi A, Mencarelli R, Belluco C, Lise M. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol. 2003;10:1077-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Riediger H, Keck T, Wellner U, zur Hausen A, Adam U, Hopt UT, Makowiec F. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg. 2009;13:1337-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 11. | Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, Vlastos G, Deglise C, Usel M, Lutz JM, Bouchardy C. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009;27:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Liu YP, Ma L, Wang SJ, Chen YN, Wu GX, Han M, Wang XL. Prognostic value of lymph node metastases and lymph node ratio in esophageal squamous cell carcinoma. Eur J Surg Oncol. 2010;36:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Falconi M, Crippa S, Domínguez I, Barugola G, Capelli P, Marcucci S, Beghelli S, Scarpa A, Bassi C, Pederzoli P. Prognostic relevance of lymph node ratio and number of resected nodes after curative resection of ampulla of Vater carcinoma. Ann Surg Oncol. 2008;15:3178-3186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Espín F, Bianchi A, Llorca S, Feliu J, Palomera E, García O, Remon J, Suñol X. Metastatic lymph node ratio versus number of metastatic lymph nodes as a prognostic factor in gastric cancer. Eur J Surg Oncol. 2012;38:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Greene FL. TNM staging for malignancies of the digestive tract: 2003 changes and beyond. Semin Surg Oncol. 2003;21:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Nitta T, Sato Y, Ren XS, Harada K, Sasaki M, Hirano S, Nakanuma Y. Autophagy may promote carcinoma cell invasion and correlate with poor prognosis in cholangiocarcinoma. Int J Clin Exp Pathol. 2014;7:4913-4921. [PubMed] |
| 17. | Wang W, Zhang J, Zhan X, Lin T, Yang M, Hu J, Han B, Hu S. SOX4 is associated with poor prognosis in cholangiocarcinoma. Biochem Biophys Res Commun. 2014;452:614-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Park KW, Jung ES, Kim DG, Yoo YK, Hong TH, Lee IS, Koh YH, Kim JH, Lee MA. ERCC1 Can Be a Prognostic Factor in Hilar Cholangiocarcinoma and Extrahepatic Bile Duct Cancer, But Not in Intrahepatic Cholangiocarcinoma. Cancer Res Treat. 2013;45:63-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Cai WK, Lin JJ, He GH, Wang H, Lu JH, Yang GS. Preoperative serum CA19-9 levels is an independent prognostic factor in patients with resected hilar cholangiocarcinoma. Int J Clin Exp Pathol. 2014;7:7890-7898. [PubMed] |
| 20. | Dong ZR, Zhang C, Cai JB, Zhang PF, Shi GM, Gao DM, Sun HC, Qiu SJ, Zhou J, Ke AW. Role of 5-hydroxymethylcytosine level in diagnosis and prognosis prediction of intrahepatic cholangiocarcinoma. Tumour Biol. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Tian X, Wang Q, Li Y, Hu J, Wu L, Ding Q, Zhang C. The expression of S100A4 protein in human intrahepatic cholangiocarcinoma: clinicopathologic significance and prognostic value. Pathol Oncol Res. 2015;21:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Murakami Y, Uemura K, Sudo T, Hashimoto Y, Kondo N, Nakagawa N, Muto T, Sasaki H, Urabe K, Sueda T. Perineural invasion in extrahepatic cholangiocarcinoma: prognostic impact and treatment strategies. J Gastrointest Surg. 2013;17:1429-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Kulig J, Sierzega M, Kolodziejczyk P, Popiela T. Ratio of metastatic to resected lymph nodes for prediction of survival in patients with inadequately staged gastric cancer. Br J Surg. 2009;96:910-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Yu JX, Li Y. The staging system of metastatic lymph node ratio in gastric cancer. Clin Oncol (R Coll Radiol). 2007;19:269-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D, Roviello F, de Manzoni G, Minicozzi A, Natalini G. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 26. | Xu DZ, Geng QR, Long ZJ, Zhan YQ, Li W, Zhou ZW, Chen YB, Sun XW, Chen G, Liu Q. Positive lymph node ratio is an independent prognostic factor in gastric cancer after d2 resection regardless of the examined number of lymph nodes. Ann Surg Oncol. 2009;16:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Kim CY, Yang DH. Adjustment of N stages of gastric cancer by the ratio between the metastatic and examined lymph nodes. Ann Surg Oncol. 2009;16:1868-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Tamandl D, Kaczirek K, Gruenberger B, Koelblinger C, Maresch J, Jakesz R, Gruenberger T. Lymph node ratio after curative surgery for intrahepatic cholangiocarcinoma. Br J Surg. 2009;96:919-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Kawai M, Tani M, Kobayashi Y, Tsuji T, Tabuse K, Horiuchi T, Oka M, Yamaguchi K, Sakata Y, Shimomura T. The ratio between metastatic and examined lymph nodes is an independent prognostic factor for patients with resectable middle and distal bile duct carcinoma. Am J Surg. 2010;199:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |