Published online Mar 28, 2015. doi: 10.3748/wjg.v21.i12.3720
Peer-review started: May 17, 2014
First decision: June 10, 2014
Revised: June 23, 2014
Accepted: July 29, 2014
Article in press: July 30, 2014
Published online: March 28, 2015
Processing time: 317 Days and 3 Hours
AIM: To evaluate the benefit and safety of sivelestat (a neutrophil elastase inhibitor) administration in patients undergoing esophagectomy.
METHODS: Online databases including PubMed, EMBASE, the Cochrane Library, Web of Knowledge, and Chinese databases (Wanfang database, VIP and CNKI) were searched systematically up to November 2013. Randomized controlled trials and high-quality comparative studies were considered eligible for inclusion. Three reviewers evaluated the methodological quality of the included studies, and Stata 12.0 software was used to analyze the extracted data. The risk ratio (RR) was used to express the effect size of dichotomous outcomes, and mean difference (MD) or standardized mean difference was used to express the effect size of continuous outcomes.
RESULTS: Thirteen studies were included in this systematic review and nine studies were included in the meta-analysis. The duration of mechanical ventilation was significantly decreased in the sivelestat group on postoperative day 5 [I2 = 76.3%, SMD = -1.41, 95%CI: -2.63-(-0.19)]. Sivelestat greatly lowered the incidence of acute lung injury in patients after surgery (I2 = 0%, RR = 0.27, 95%CI: 0.08-0.93). However, it did not decrease the incidence of pneumonia, intensive care unit stay or postoperative hospital stay, and did not increase the incidence of complications such as anastomotic leakage, recurrent nerve palsy, wound infection, sepsis and catheter-related fever.
CONCLUSION: A neutrophil elastase inhibitor is beneficial in patients undergoing esophagectomy. More high quality, large sample, multi-center and randomized controlled trials are needed to validate this effect.
Core tip: Radical esophagectomy has been adopted in patients with esophageal carcinoma to improve survival. This technique is highly invasive, leading to excess surgical stress, a perioperative mortality of 3%-10%, and pulmonary disorders account for nearly 30%-60%. Sivelestat sodium hydrate, a specific neutrophil elastase inhibitor, actively protects patients with acute respiratory diseases. The efficacy and safety of sivelestat administered during esophagectomy has produced conflicting results and the conclusions from relevant studies are presented. This meta-analysis revealed that sivelestat is beneficial in patients undergoing esophagectomy, especially in terms of the duration of mechanical ventilation and the incidence of pulmonary complications.
- Citation: Wang ZQ, Chen LQ, Yuan Y, Wang WP, Niu ZX, Yang YS, Cai J. Effects of neutrophil elastase inhibitor in patients undergoing esophagectomy: A systematic review and meta-analysis. World J Gastroenterol 2015; 21(12): 3720-3730
- URL: https://www.wjgnet.com/1007-9327/full/v21/i12/3720.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i12.3720
Esophageal carcinoma is the sixth leading cause of cancer-related deaths worldwide, and its incidence is increasing rapidly[1,2]. In recent years, multidisciplinary treatments have been adopted more and more frequently. Of these treatments, curative surgery remains the most important treatment option[3,4]. Previous studies have shown that patients undergoing radical esophagectomy after neoadjuvant therapy achieved the highest long-term survival[4-6].
Radical esophagectomy, which consists of video-assisted thoracoscopic esophagectomy, cervical esophagogastrostomy and two- or three-field lymph node dissection, is one of the most invasive surgical techniques performed in the gastrointestinal system[7]. This excess surgical stress has led to a perioperative mortality rate of approximately 3%-10%[8,9], and is mainly caused by systemic inflammatory response syndrome (SIRS)-associated complications, of which pulmonary disorders account for approximately 30%-60%[10].
The lung is the main target organ for overproduced cytokines in SIRS; thus, pneumonia, acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) occur frequently in patients undergoing esophagectomy[11,12]. Current studies have demonstrated that neutrophil elastase (NE), which is secreted by IL-8 induced mature neutrophils, could represent the severity of postoperative pulmonary disorders[13]. In addition, Suda et al[14] stated that a drug that could relieve SIRS and control neutrophil function might improve the postoperative clinical course following transthoracic esophagectomy.
Sivelestat sodium hydrate, a synthetic NE inhibitor, can competitively inhibit NE activity and does not affect other proteases[15]. A positive treatment effect was reported in many studies, and the Japanese Respiratory Society recommends sivelestat for the treatment of ALI in the Guidelines for Treatment of ALI/ARDS[16]. However, reports on the benefits of sivelestat administration during esophagectomy in patients with esophageal carcinoma have shown conflicting results[17-19]. It is not known whether sivelestat can improve the postoperative clinical course, reduce lung function damage, and alter blood, cytokine and lung injury markers. Although some traditional reviews exist, the data from these reviews are not comprehensive and are insufficient. Therefore, we performed a systematic review and meta-analysis to evaluate the benefit and safety of sivelestat administration in patients undergoing esophagectomy.
Online databases, including PubMed, EMBASE, the Cochrane Library, Web of Knowledge, and Chinese databases (Wanfang database, VIP and CNKI) were searched systematically and comprehensively up to November 2013. In addition, clinicalTrials.gov and recent conferences were also searched. Search terms were “esophageal cancer OR esophagectomy” in combination with “neutrophil elastase inhibitor OR sivelestat OR sivelestat sodium OR frese lestat” without limitation of publication year, status and language. Review articles were also scanned to identify relevant studies by reading the reference list.
Randomized controlled trials and high-quality comparative studies were considered eligible for inclusion if: (1) the participants were esophageal carcinoma patients undergoing esophagectomy; (2) neutrophil elastase inhibitor was compared with placebo (saline); and (3) outcomes mainly included data on postoperative clinical course, oxygenation, blood and cytokines. Studies on patients undergoing other major surgeries were excluded. Quantitative data were not necessary for inclusion. According to the inclusion criteria, two reviewers independently reviewed the searched literature and any disagreement was resolved by discussion.
Data were extracted and a form, which was devised in advance, was completed. The following data were recorded: basic information (author, country and year of publication), characteristics (sex, age and arm), treatment protocol (case, sivelestat dosage and usage), surgical background (operative time, blood loss, surgical procedure), outcome measures [duration of mechanical ventilation, intensive care unit (ICU) stay, SIRS, postoperative hospital stay, and P/F ratio], and complications. Another two reviewers carried out the data extraction, and the results were then cross-checked. Disagreement was resolved by discussion.
Three reviewers evaluated the methodological quality of the included studies according to the standard recommended by the Cochrane handbook[20] for systematic reviews and meta-analyses. By studying the materials and methods section, quality assessment was performed by identifying the study type, randomization, blinding, allocation concealment, eligibility criteria, baseline comparability, participants lost to follow-up, ITT analysis, selective reporting, incomplete outcome and other biases.
Stata 12.0 software was used to analyze the extracted data. The risk ratio (RR) was used to express the effect size of dichotomous outcomes, and the mean difference (MD) or standardized mean difference was used to express the effect size of continuous outcomes. Cochran’s Q-test and the I2 statistic were used to estimate the heterogeneity among the pooled studies. If P > 0.05 or I2 < 50%, the heterogeneity was thought to be insignificant, and a fixed-effect model was adopted in the meta-analysis. If the heterogeneity was significant, a random-effect model was adopted and the source of heterogeneity was investigated using clinical and statistical aspects. In addition, sensitivity was assessed to judge the reliability of the evidence, and both Begg’s test and Egger’s test were conducted to determine publication bias.
This review was performed in accordance with The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The present protocol has not been published or registered elsewhere.
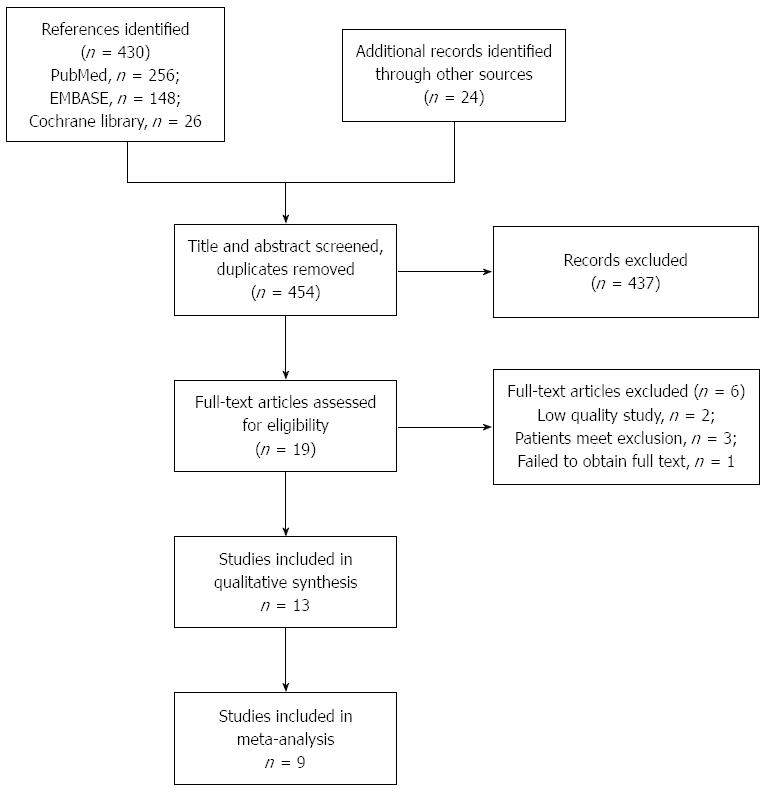
The flowchart of the trial selection is shown in Figure 1. A total of 454 references were identified from the online databases and other sources, and after screening the title and abstract, 17 references were selected for full-text assessment. In total, 13 studies were included in this systematic review[14,19-30] and nine studies were included in the meta-analysis[14,19,20,22,24-28].
Tables 1 and 2 describe the baseline and basic information on the included studies. Ten studies had two arms: sivelestat-treated arm and saline-treated or control arm, and one study[21] had three arms: two sivelestat-treated arms and a control arm. All the studies were performed in Japan, with 10 published in English and one published in Japanese. Other information, such as the sex and age of participants, dosage and usage of sivelestat, and surgical procedure related indices are summarized in detail, and all showed no significant differences between the treatment group and control group. Table 3 shows the results of the methodological quality assessment, which was carried out according to the methods recommended by The Cochrane Collaboration.
| Ref. | Year | Arm | Case (n) | Sex (M/F) | Age (yr) | Usage of sivelestat |
| Sato et al[19] | 2001 | SSH | 8 | - | 63.9 ± 6.9 | 150000 U diluted in 20 mL normal saline every 12 h from operation to POD 5 |
| Saline | 8 | - | 64.6 ± 8.7 | |||
| Akamoto et al[20] | 2007 | SSH | 6 | 5/1 | 70.8 ± 5.5 | 4.8 mg/kg per day of sivelestat + 240 mL saline from operation to POD 3 |
| Saline | 7 | 5/2 | 65.7 ± 2.9 | |||
| Kawahara et al[22] | 2010 | SSH | 10 | 7/3 | 64 (50-78)1 | 300 mg/d of sivelestat + 200 mL saline from operation to POD 3 |
| Saline | 10 | 10/0 | 63 (65-69) | |||
| Makino et al[24] | 2011 | SSH | 16 | 12/4 | 65 (61-68)2 | 4.8 mg/kg per day of sivelestat + 240 mL saline from operation to POD 7 |
| Saline | 15 | 13/2 | 66 (63-69) | |||
| Yamaguchi et al[29] | 2011 | SSH | 12 | 9/3 | 59 ± 5 | 0.2 mg/kg per hour sivelestat from operation to POD 1 |
| Saline | 12 | 9/3 | 60 ± 8 | |||
| Iwahashi et al[21] | 2011 | Arm1 | 15 | 13/2 | 65 ± 8 | Arm1: 0.2 mg/kg per hour sivelestat from operation to POD 1; |
| Arm2 | 15 | 9/6 | 64 ± 7 | Arm 2: 0.2 mg/kg per hour sivelestat from operation to POD 5 | ||
| Control | 15 | 10/5 | 67 ± 8 | 0.2 mg/kg per hour sivelestat | ||
| Yamaki et al[30] | 2005 | SSH | 9 | - | 62 ± 9 | |
| Control | 6 | - | 69 ± 8 | 0.2 mg/kg per hour sivelestat after operation till POD 5 | ||
| Ono et al[28] | 2007 | SSH | 7 | 4/3 | 61 ± 12 | |
| Control | 10 | 7/3 | 70 ± 7 | 0.2 mg/kg per hour sivelestat diluted with saline after operation till POD 6 | ||
| Suda et al[14] | 2007 | SSH | 18 | 15/3 | 60 (55-65)3 | |
| Control | 25 | 20/5 | 56 (52-66) | 0.2 mg/kg per hour from operation and during mechanical ventilation support | ||
| Kobayashi et al[23] | 2010 | SSH | 60 | 56/4 | 66 ± 7 | |
| Control | 28 | 24/4 | 60 ± 10 | 0.2 mg/kg per hour sivelestat diluted with saline after operation till POD 5 | ||
| Mimatsu et al[25] | 2011 | SSH | 22 | 21/1 | 59 ± 11 | |
| Control | 20 | 19/1 | 63 ± 9 | 0.2 mg/kg per hour sivelestat after operation till POD 3 | ||
| Nishiyama et al[27] | 2012 | SSH | 26 | 23/3 | 67 ± 8 | |
| Control | 27 | 23/4 | 63 ± 8 | 0.2 mg/kg per hour sivelestat with 5% dextrose in water from operation till POD 3 | ||
| Nagai et al[26] | 2013 | SSH | 42 | 39/3 | 66 ± 9 | |
| Control | 35 | 31/4 | 63 ± 8 |
| Ref. | Arm | Operative time (min) | Blood loss (mL) | Surgical procedure |
| Sato et al[19] | SSH | 357 ± 58 | 615 ± 268 | Extensive resection including lymph node dissection |
| Saline | 326 ± 23 | 712 ± 184 | ||
| Akamoto et al[20] | SSH | 496 ± 140 | 1 672 ± 426 | Esophagectomy and esophagogastric anastomosis |
| Saline | 569 ± 46 | 1 339 ± 316 | ||
| Kawahara et al[22] | SSH | 517 (range 443-733) | 305 (range 180-1050) | Video-assisted thoracoscopic oesophagectomy |
| Saline | 549 (range 453-785) | 32 (range 150-1910) | ||
| Makino et al[24] | SSH | 433 (95%CI: 399-467) | 468 (95%CI: 380-556) | Video-assisted thoracoscopic oesophagectomy |
| Saline | 431 (95%CI: 407-455) | 514 (95%CI: 386-643) | ||
| Yamaguchi et al[29] | SSH | 387 ± 57 | 488 ± 229 | Right-sided transthoracic esophagectomy with cervical esophagogastrostomy and lymph node dissection |
| Saline | 363 ± 85 | 376 ± 166 | ||
| Iwahashi et al[21] | SSH | 491 ± 62 | 422 ± 210 | Radical esophagectomy with a two- or three-field lymph node dissection via a cervicothoracoabdominal approach |
| SSH | 466 ± 72 | 405 ± 262 | ||
| Control | 482 ± 69 | 430 ± 173 | ||
| Yamaki et al[30] | SSH | 538 ± 121 | 969 ± 505 | Radical esophagectomy |
| Control | 552 ± 157 | 1134 ± 682 | ||
| Ono et al[28] | SSH | 573.4 ± 72.6 | 1685.1 ± 1255.3 | Esophagectomy and reconstruction with gastric mobilization by right posterolateral thoracotomy and laparotomy |
| Control | 568.7 ± 164.1 | 1032.4 ± 347.7 | ||
| Suda et al[14] | SSH | 458 (95%CI: 373-545) | 361 (95%CI: 218-682) | Transthoracic esophagectomy |
| Control | 626 (95%CI: 541-700) | 520 (95%CI: 216-700) | ||
| Kobayashi et al[23] | SSH | 311 ± 66 | 359 ± 253 | Thoracoscopy-assisted subtotal esophagectomy |
| Control | 412 ± 71 | 402 ± 161 | ||
| Mimatsu et al[25] | SSH | 407.3 ± 74.6 | 346.7 ± 122.2 | Transthoracic esophagectomy with reconstruction of the stomach role via the posterior sternum |
| Control | 396.7 ± 96.3 | 354.4 ± 134.5 | ||
| Nishiyama et al[27] | SSH | 450.2 ± 64.1 | 813.6 ± 548.4 | Thoracolaparotomic total thoracic esophagectomy, chest wall-antral stomach reconstruction, and 3-regional lymph node dissection |
| Control | 445.8 ± 87.9 | 735.2 ± 479.0 | ||
| Nagai et al[26] | SSH | 576.4 ± 126.7 | 630.1 ± 392.0 | Subtotal esophagectomy and reconstruction through a right posterolateral thoracotomy and upper midline laparotomy |
| Control | 537.3 ± 120.2 | 494.2 ± 312.7 |
| Ref. | Type | Randomization | Blinding | Allocation concealment | Eligibility criteria | Baseline comparability | > 85% participants followed up | ITT analysis | Selective reporting | Incomplete outcome | Other bias |
| Sato et al[19] | RCT | M | U | U | Y | Y | Y | Y | U | N | U |
| Akamoto et al[20] | RCT | Y | Y, single blinding | U | Y | Y | Y | Y | U | N | U |
| Kawahara et al[22] | RCT | M | M, double blinding | U | Y | Y | Y | Y | U | N | U |
| Makino et al[24] | RCT | Y | Y, triple blinding | Y | Y | Y | Y | Y | U | N | U |
| Yamaguchi et al[29] | RCT | M | U | U | Y | Y | Y | Y | U | U | U |
| Iwahashi et al[21] | non-RCT | N | U | U | Y | Y | Y | N | U | N | U |
| Yamaki et al[30] | non-RCT | N | N | N | M | Y | Y | U | U | N | U |
| Ono et al[28] | non-RCT | N | N | N | Y | Y | Y | Y | U | N | U |
| Suda et al[14] | non-RCT | N | N | N | Y | Y | Y | Y | U | N | U |
| Kobayashi et al[23] | non-RCT | N | N | N | Y | Y | Y | Y | N | N | U |
| Mimatsu et al[25] | non-RCT | N | N | N | Y | Y | Y | Y | N | N | U |
| Nishiyama et al[27] | non-RCT | N | N | N | Y | Y | Y | Y | N | N | U |
| Nagai et al[26] | non-RCT | N | N | N | M | Y | Y | Y | U | N | U |
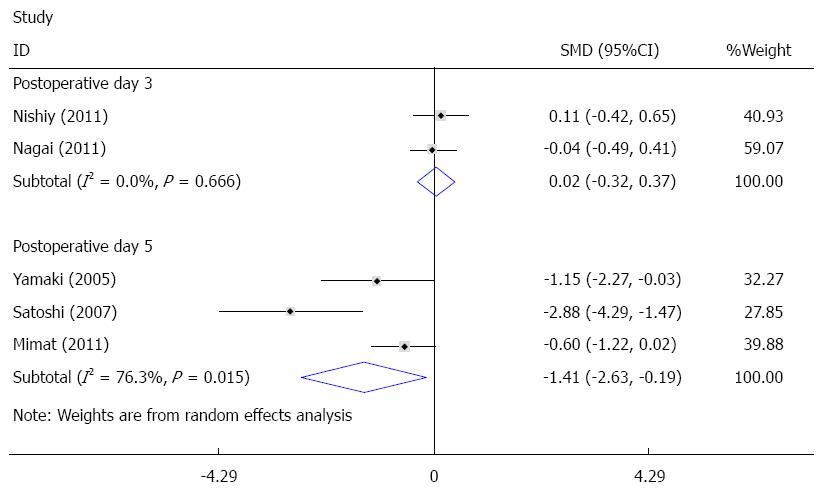
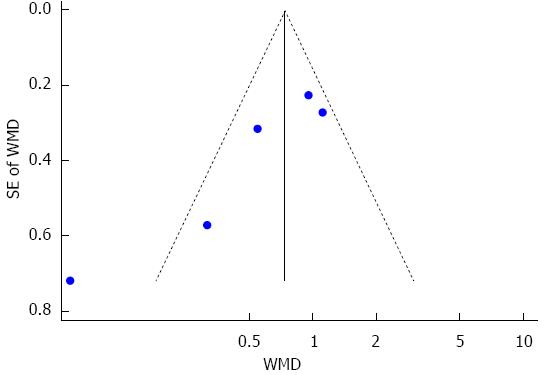
The duration of mechanical ventilation was reported in eight studies[14,22,24-28,30], and five of these studies were pooled quantitatively in this meta-analysis[25-28,30]. There was significant heterogeneity among the trials. To investigate the source of heterogeneity, according to postoperative day (POD) of sivelestat administration, subgroup analysis including POD 3 (sivelestat was administrated until POD3 and POD 5 (sivelestat was administrated until POD 5) was performed. When compared with the control group, the duration of mechanical ventilation was significantly decreased in the sivelestat group on POD 5 [I2 = 76.3%, SMD = -1.41, 95%CI: -2.63-(-0.19)]. Although the duration of mechanical ventilation was also decreased in the sivelestat group on POD 3, it failed to reach statistical significance (I2 = 0%, SMD= -0.68, 95%CI:-1.38-0.02). Begg’s test and Egger’s test showed that publication bias might exist (P = 0.027, 95%CI: -8.82-1.06). These data are shown in Figures 2 and 3. The data in the other three studies are summarized in Table 4.
| Study | Kawah et al1 | Makino et al2 | Suda et al3 | |||
| SSHvscontrol | P value | SSHvscontrol | P value | SSHvscontrol | P value | |
| Mechanical ventilation | 24.5 (24.3-28.7) vs 24.5 (23.9-49.1) | 0.796 | 89.5 (57.3, 121.7) vs 204 (77.4, 330.6) | 0.046 | 1 (1-1.5) vs 1.5 (1-2) | 0.008 |
| ICU stay | 64.0 (39-109) vs 74.5 (39.0-109) | 0.481 | 5.7 (4.1, 7.4) vs 8.8 (5.5, 12.1) | 0.048 | 1.5 (1.5-1.9) vs 2.5 (1.5-3.5) | 0.018 |
| SIRS | 17 (9-36) vs 49 (15-60) | 0.009 | 2.8 (2.1, 3.6) vs 5.6 (4.2,7.0 | 0.001 | 3.5 (2-5.8) vs 5 (3.8-10.8) | 0.026 |
| Postoperative hospital stay | 32 (19-46) vs 31 (18-81) | 0.853 | 31.4 (23.8, 38.9) vs 37.1 (31.1, 43.1) | 0.077 | ||
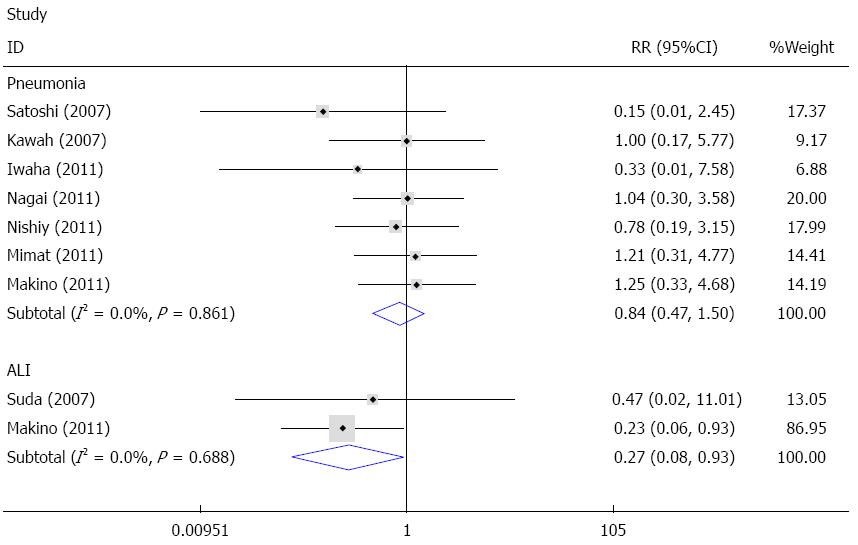
Pneumonia and ALI were common pulmonary complications after esophagectomy. Seven studies reported data on pneumonia[21,22,24-28], and the fixed effects meta-analysis showed that sivelestat decreased the incidence of pneumonia compared with the control; however, the difference was not statistically significant (I2 = 0%, RR = 0.84, 95%CI: 0.47-1.50). Available data on ALI was reported in two studies[14,24]. The fixed effects model meta-analysis demonstrated that sivelestat greatly decreased the incidence of ALI in patients after surgery (I2 = 0%, RR = 0.27, 95%CI: 0.08-0.93). Begg’s test and Egger’s test indicated that no publication bias existed (P = 0.214, 95%CI: -3.24-0.87). These data are shown in Figures 4 and 5.
Five studies presented data on SIRS[14,22,24-26], and these studies demonstrated that sivelestat decreased the duration of SIRS. Of these five studies, four[14,22,24,25] stated that there were significant differences between the sivelestat group and the control group (P = 0.046, P = 0.048, P = 0.018, P = 0.048), but one[26] stated that the difference failed to reach statistical significance (P > 0.05), as shown in Table 4.
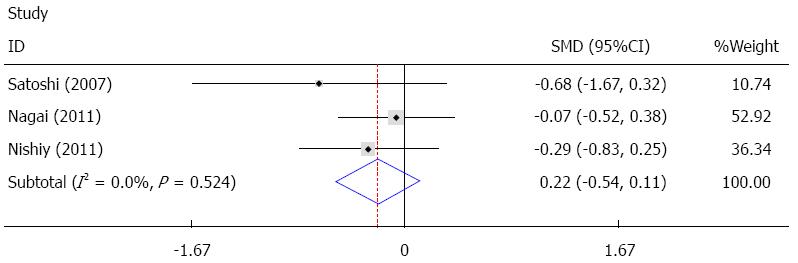
Six studies provided data on ICU stay[14,22,24,26-28], and three of these studies were pooled quantitatively in the fixed effect analysis[26-28]. The results showed that sivelestat decreased ICU stay, but this failed to achieve statistical significance [I2 = 0%, SMD = -0.22, 95%CI: -0.54-(-0.11)], as shown in Figure 6. In the other three studies, one study reported no statistically significant difference, and two studies found a statistically significant difference between the sivelestat group and the control group, as summarized in Table 4.
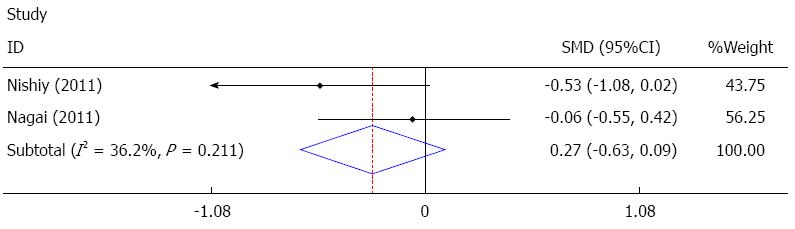
Postoperative hospital stay was reported in four studies[22,24,26,27], and two of these studies[26,27] were pooled quantitatively in the fixed effect analysis. The results showed that sivelestat decreased postoperative hospital stay, but it failed to achieve statistical significance [I2 = 36.2%, SMD = -0.27, 95%CI: -0.63-(-0.09)], as shown in Figure 7. The other two studies[22,24] showed no significant difference, as summarized in Table 4.
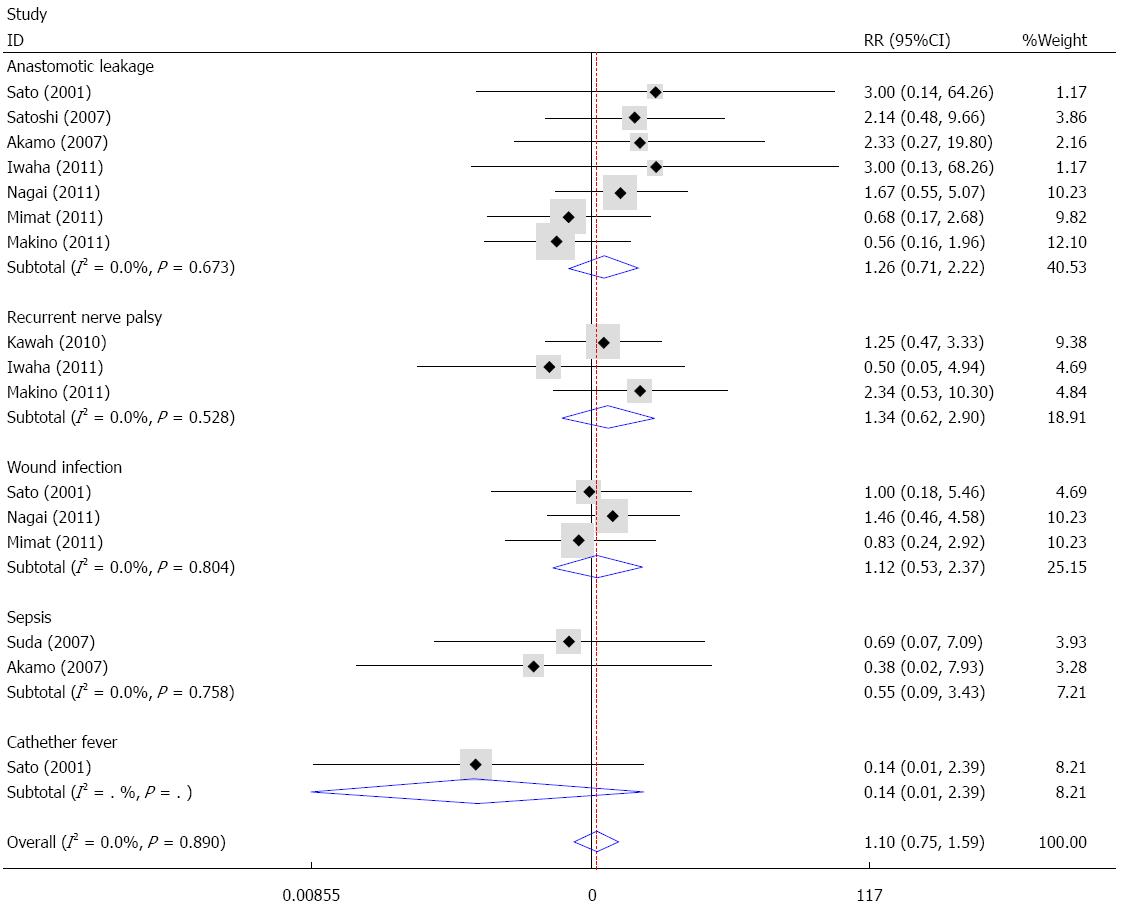
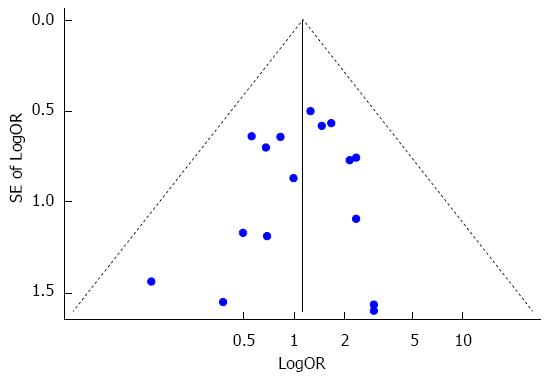
With the exception of pulmonary complications, other complications such as anastomotic leakage, recurrent nerve palsy, wound infection and sepsis were also reported in the included studies. Fixed effects analysis demonstrated that no significant difference existed between the sivelestat group and the control group in terms of anastomotic leakage (I2 = 0%, RR = 1.26, 95%CI: 0.71-2.22), recurrent nerve palsy (I2 = 0%, RR = 1.34, 95%CI: 0.62-2.90), wound infection (I2 = 0%, RR = 1.12, 95%CI: 0.53-2.37), sepsis (I2 = 0%, RR = 0.55, 95%CI: 0.09-3.43) and catheter-related fever (RR = 0.14, 95%CI: 0.01-2.39). Overall, sivelestat did not significantly increase the incidence of these complications (I2 = 0%, P = 1.10, 95%CI: 0.75-1.59), and Begg’s test and Egger’s test indicated that no publication bias existed (P = 0.53, 95%CI: -1.57-0.84). These data are shown in Figures 8 and 9.
Some studies have found that patients undergoing esophagectomy benefit from methylprednisolone administration with no adverse effects. However, even when pre-operative methylprednisolone is administered, pulmonary complications frequently occur. This may be caused by the systemic inflammatory response following esophagectomy, leading to accumulation of neutrophils in the lungs. Subsequent local release of neutrophil elastase (NE) injures the lung[18,31]. As glucocorticoids do not affect the release or function of NE, additional selective inhibition of NE might be beneficial. Indeed, the results of the meta-analysis showed that compared with the control group, the duration of mechanical ventilation support was reduced in the sivelestat group. Subgroup analysis demonstrated that this reduction in the duration of mechanical ventilation support failed to reach statistical significance in the sivelestat group on POD 3, but it was significantly decreased in the sivelestat group on POD 5. Our study revealed that sivelestat administered at different times may lead to different clinical outcomes, and the administration of sivelestat should be continued up to at least POD 5 to decrease the time required for mechanical ventilation support.
Pneumonia and ALI are common pulmonary complications after esophagectomy[10], and our results indicated that although sivelestat may not decrease the incidence of pneumonia compared with the control, it greatly reduced the incidence of ALI in patients after surgery. Although ARDS and SIRS have been clearly defined during the American-European consensus conferences, the criteria for pneumonia differ widely[32]. Consequently, the study results for pneumonia should be considered with caution. Furthermore, pneumonia after esophagectomy can be caused by various factors such as increased infection, invasive surgical procedures, administration of methylprednisolone, decreased pulmonary function and immunity, and the use of mechanical ventilation support[33]. ALI mainly occurs because of increased levels of cytokines in the serum, especially NE secreted by neutrophils. Thus, as a specific inhibitor of NE, sivelestat, had a very limited effect on postoperative pneumonia, but a very strong effect on postoperative ALI. In addition, sivelestat had a positive effect on pulmonary function. Kawaha et al[22] reported a significant increase in PaO2/FiO2 on POD 1 and 7; Suda et al[14] reported a significant increase in PaO2/FiO2 on POD 1; and Nishiyama et al[27] reported a significant increase in PaO2 on POD 5.
Most studies reported a reduction in the duration of postoperative SIRS; however, two studies found no statistically significant difference[21,26]. Of these two studies, Iwahashi et al[21] performed esophagectomy using the cervicothoracoabdominal approach, and Nagai et al[26] performed subtotal esophagectomy via a right posterolateral thoracotomy and upper midline laparotomy. Compared with current video-assisted thoracoscopic esophagectomy[34,35], their surgical procedures appeared to be more invasive and led to more blood loss, which induced a more acute SIRS state. Therefore, additional sivelestat administration after the more invasive surgical procedure may have little clinical benefit, and the effects of different procedures in addition to higher dose of sivelestat should be investigated in the future.
The meta-analysis results showed that sivelestat may have decreased ICU stay; however, this decrease failed to achieve statistical significance. In the other three studies mentioned previously, two studies[14,24] reported significant differences, while one study[22] reported no significant difference, thus there is no consensus on ICU stay. Postoperative hospital stay was reported in four studies[22,24,26,27], and only two of these studies[26,27] were pooled quantitatively in the fixed effect analysis. The results showed that sivelestat might have decreased postoperative hospital stay; however, this decrease failed to achieve statistical significance. The other two studies[22,24] showed no significant difference. With sivelestat administrated after surgery, the mechanical ventilation support, pulmonary complications and SIRS were improved; however, the ICU stay and postoperative hospital stay were not significantly shortened. Possible explanations for these findings are as follows: (1) limited number of studies included in the analysis; (2) insufficient data in the studies; (3) different protocols for discharging from the ICU and hospital adopted in the studies; (4) heterogeneity between the studies; and (5) different protocols of sivelestat administration.
One study performed a cost-analysis[27], which showed that only surgery costs were significantly lower in the sivelestat group compared with the control group, and there were no significant differences in the hospitalization, medication or total costs. Therefore, additional sivelestat did not increase medical costs. With regard to safety, our study demonstrated that sivelestat did not increase the risk of complications, including anastomotic leakage, recurrent nerve palsy, wound infection and sepsis.
There are also some weaknesses with the present evidence. Some of the included trials were non-RCTs, which may have increased the risk of random errors. Dissimilar procedures, such as minimally invasive or traditional surgery with different operative time and blood loss, could affect patient outcomes. In addition, different concentrations of sivelestat administered with inconsistent doses of methylprednisolone may decrease the risk of pulmonary complications. All of these factors suggest that there may be unavoidable bias in the pooled results, which in turn limited the strength of this meta-analysis. Minimally invasive surgery has evolved rapidly in recent years. As minimally invasive approaches reduce the factors associated with pulmonary complications (e.g., blood loss, pain and inflammation), minimally invasive esophagectomy would be particularly beneficial with respect to pulmonary complications. In the included studies, three studies performed thoracoscopy-assisted surgery[22-24], and two studies performed subtotal esophagectomy[23,26]; therefore, the different procedures adopted in these studies would also have some effect on the results.
The results for perioperatively administered neutrophil elastase inhibitor are encouraging. All the trials included were conducted in Eastern populations, and genomic factors may have influenced the results[36,37]. Further trials are required in other areas to determine whether these results can be extrapolated to all populations.
In summary, neutrophil elastase inhibitor administration is beneficial in patients undergoing esophagectomy, especially in terms of the duration of mechanical ventilation, pulmonary function, pulmonary complications and SIRS state. Although many studies have reported that it also plays an active role in ICU stay and hospital stay, there is currently insufficient evidence for these effects, and more high-quality, large sample, multi-center and randomized controlled trials are needed.
Esophageal carcinoma is the sixth leading cause of cancer-related deaths worldwide. Patients undergoing radical esophagectomy suffer excess surgical stress, which mainly causes pulmonary complications. Sivelestat sodium hydrate is recommended for the treatment of acute lung injury, and is considered effective in patients with esophageal carcinoma undergoing esophagectomy. However, this needs to be systematically evaluated.
This meta-analysis was performed to evaluate the benefit and safety of sivelestat administration in patients undergoing esophagectomy. The outcome measures included mechanical ventilation, pulmonary complications, SIRS, ICU stay, postoperative hospital stay and other complications.
This meta-analysis revealed that sivelestat is beneficial in patients undergoing esophagectomy, especially in terms of the duration of mechanical ventilation and the incidence of pulmonary complications. It may also play an active role on ICU stay, hospital stay, oxygenation and blood cytokine levels. However, there is currently insufficient evidence for these effects.
The current analysis shows that sivelestat sodium hydrate may achieve better treatment outcomes in patients with esophageal carcinoma undergoing esophagectomy. Sivelestat reduced the duration of mechanical ventilation support and the incidence of pulmonary complications. In addition, side effects did not appear to be a concern.
Radical esophagectomy, which mainly consists of video-assisted thoracoscopic esophagectomy, cervical esophagogastrostomy and two- or three-field lymph node dissection, is one of the most invasive surgical techniques performed in the gastrointestinal system.
This is a nicely written manuscript with a thoroughly performed review and meta-analysis on the use of sivelestat perioperatively for esophagectomy, and the outcomes and analyses were really conducive.
P- Reviewer: Li H, Nickel F S- Editor: Qi Y L- Editor: Stewart G E- Editor: Ma S
| 1. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2115] [Cited by in F6Publishing: 2168] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25335] [Article Influence: 1948.8] [Reference Citation Analysis (7)] |
| 3. | Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727-1733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1121] [Cited by in F6Publishing: 1060] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 4. | Hofstetter W, Swisher SG, Correa AM, Hess K, Putnam JB, Ajani JA, Dolormente M, Francisco R, Komaki RR, Lara A. Treatment outcomes of resected esophageal cancer. Ann Surg. 2002;236:376-84; discussion 384-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 5. | Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van Dekken H, Obertop H. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662-1669. [PubMed] [Cited in This Article: ] |
| 6. | Law S, Kwong DL, Kwok KF, Wong KH, Chu KM, Sham JS, Wong J. Improvement in treatment results and long-term survival of patients with esophageal cancer: impact of chemoradiation and change in treatment strategy. Ann Surg. 2003;238:339-47; discussion 347-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Haga Y, Beppu T, Doi K, Nozawa F, Mugita N, Ikei S, Ogawa M. Systemic inflammatory response syndrome and organ dysfunction following gastrointestinal surgery. Crit Care Med. 1997;25:1994-2000. [PubMed] [Cited in This Article: ] |
| 8. | Avendano CE, Flume PA, Silvestri GA, King LB, Reed CE. Pulmonary complications after esophagectomy. Ann Thorac Surg. 2002;73:922-926. [PubMed] [Cited in This Article: ] |
| 9. | Bailey SH, Bull DA, Harpole DH, Rentz JJ, Neumayer LA, Pappas TN, Daley J, Henderson WG, Krasnicka B, Khuri SF. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg. 2003;75:217-22; discussion 222. [PubMed] [Cited in This Article: ] |
| 10. | Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 633] [Cited by in F6Publishing: 601] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 11. | Morita M, Yoshida R, Ikeda K, Egashira A, Oki E, Sadanaga N, Kakeji Y, Ichiki Y, Sugio K, Yasumoto K. Acute lung injury following an esophagectomy for esophageal cancer, with special reference to the clinical factors and cytokine levels of peripheral blood and pleural drainage fluid. Dis Esophagus. 2008;21:30-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Tandon S, Batchelor A, Bullock R, Gascoigne A, Griffin M, Hayes N, Hing J, Shaw I, Warnell I, Baudouin SV. Peri-operative risk factors for acute lung injury after elective oesophagectomy. Br J Anaesth. 2001;86:633-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Duswald KH, Jochum M, Schramm W, Fritz H. Released granulocytic elastase: an indicator of pathobiochemical alterations in septicemia after abdominal surgery. Surgery. 1985;98:892-899. [PubMed] [Cited in This Article: ] |
| 14. | Suda K, Kitagawa Y, Ozawa S, Miyasho T, Okamoto M, Saikawa Y, Ueda M, Yamada S, Tasaka S, Funakoshi Y. Neutrophil elastase inhibitor improves postoperative clinical courses after thoracic esophagectomy. Dis Esophagus. 2007;20:478-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Yoshikawa N, Inomata T, Okada Y, Shimbo T, Takahashi M, Akita K, Uesugi Y, Narumi Y. Sivelestat sodium hydrate reduces radiation-induced lung injury in mice by inhibiting neutrophil elastase. Mol Med Rep. 2013;7:1091-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Hayakawa M, Katabami K, Wada T, Sugano M, Hoshino H, Sawamura A, Gando S. Sivelestat (selective neutrophil elastase inhibitor) improves the mortality rate of sepsis associated with both acute respiratory distress syndrome and disseminated intravascular coagulation patients. Shock. 2010;33:14-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Matsuzaki K, Hiramatsu Y, Homma S, Sato S, Shigeta O, Sakakibara Y. Sivelestat reduces inflammatory mediators and preserves neutrophil deformability during simulated extracorporeal circulation. Ann Thorac Surg. 2005;80:611-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Tamakuma S, Ogawa M, Aikawa N, Kubota T, Hirasawa H, Ishizaka A, Taenaka N, Hamada C, Matsuoka S, Abiru T. Relationship between neutrophil elastase and acute lung injury in humans. Pulm Pharmacol Ther. 2004;17:271-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Sato N, Endo S, Kimura Y, Ikeda K, Aoki K, Iwaya T, Akiyama Y, Noda Y, Saito K. Influence of a human protease inhibitor on surgical stress induced immunosuppression. Dig Surg. 2002;19:300-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Akamoto S, Okano K, Sano T, Yachida S, Izuishi K, Usuki H, Wakabayashi H, Suzuki Y. Neutrophil elastase inhibitor (sivelestat) preserves antitumor immunity and reduces the inflammatory mediators associated with major surgery. Surg Today. 2007;37:359-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Iwahashi M, Nakamori M, Nakamura M, Ojima T, Naka T, Yamaue H. Optimal period for the prophylactic administration of neutrophil elastase inhibitor for patients with esophageal cancer undergoing esophagectomy. World J Surg. 2011;35:1573-1579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Kawahara Y, Ninomiya I, Fujimura T, Funaki H, Nakagawara H, Takamura H, Oyama K, Tajima H, Fushida S, Inaba H. Prospective randomized controlled study on the effects of perioperative administration of a neutrophil elastase inhibitor to patients undergoing video-assisted thoracoscopic surgery for thoracic esophageal cancer. Dis Esophagus. 2010;23:329-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Kobayashi M, Irinoda T, Akiyama Y, Meguro E, Hayakawa Y, Funato O, Takagane A. Effect of a selective neutrophil elastase inhibitor on early recovery from body water imbalance after transthoracic esophagectomy. Dis Esophagus. 2010;23:565-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Makino H, Kunisaki C, Kosaka T, Akiyama H, Morita S, Endo I. Perioperative use of a neutrophil elastase inhibitor in video-assisted thoracoscopic oesophagectomy for cancer. Br J Surg. 2011;98:975-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Mimatsu K, Oida T, Kawasaki A, Kano H, Kuboi Y, Amano S. Influence of neutrophil elastase inhibitor on the postoperative course in patients with esophageal cancer after transthoracic esophagectomy. Hepatogastroenterology. 2011;58:1583-1587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 26. | Nagai Y, Watanabe M, Baba Y, Iwatsuki M, Hirashima K, Karashima R, Kurashige J, Kinoshita K, Baba H. Preventive effect of sivelestat on postoperative respiratory disorders after thoracic esophagectomy. Surg Today. 2013;43:361-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Nishiyama J, Matsuda M, Ando S, Hirasawa M, Suzuki T, Makuuchi H. The effects of the early administration of sivelestat sodium, a selective neutrophil elastase inhibitor, on the postoperative course after radical surgery for esophageal cancer. Surg Today. 2012;42:659-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Ono S, Tsujimoto H, Hiraki S, Takahata R, Kimura A, Kinoshita M, Ichikura T, Mochizuki H. Effects of neutrophil elastase inhibitor on progression of acute lung injury following esophagectomy. World J Surg. 2007;31:1996-2001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Yamaguchi K, Sugasawa Y, Takeuchi K, Kugimiya T, Kumakura S, Iwanuma Y, Kajiyama Y, Tsurumaru M, Nagaoka I, Inada E. Effects of sivelestat on bronchial inflammatory responses after esophagectomy. Int J Mol Med. 2011;28:187-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Yamaki E, Ogata K, Hinohara H, Kadoi Y, Kunimoto F, Kuwano H. [Effects of neutrophil elastase inhibitor on postoperative cytokine levels in patients after esophagectomy]. Masui. 2005;54:884-888. [PubMed] [Cited in This Article: ] |
| 31. | Kawabata K, Hagio T, Matsumoto S, Nakao S, Orita S, Aze Y, Ohno H. Delayed neutrophil elastase inhibition prevents subsequent progression of acute lung injury induced by endotoxin inhalation in hamsters. Am J Respir Crit Care Med. 2000;161:2013-2018. [PubMed] [Cited in This Article: ] |
| 32. | Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M, Bruce N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ. 2008;86:390-398C. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 307] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 33. | Low DE, Bodnar A. Update on clinical impact, documentation, and management of complications associated with esophagectomy. Thorac Surg Clin. 2013;23:535-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Osugi H, Takemura M, Higashino M, Takada N, Lee S, Kinoshita H. A comparison of video-assisted thoracoscopic oesophagectomy and radical lymph node dissection for squamous cell cancer of the oesophagus with open operation. Br J Surg. 2003;90:108-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 160] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Osugi H, Takemura M, Higashino M, Takada N, Lee S, Ueno M, Tanaka Y, Fukuhara K, Hashimoto Y, Fujiwara Y. Video-assisted thoracoscopic esophagectomy and radical lymph node dissection for esophageal cancer. A series of 75 cases. Surg Endosc. 2002;16:1588-1593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Meyer NJ. Future clinical applications of genomics for acute respiratory distress syndrome. Lancet Respir Med. 2013;1:793-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |