Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. Feb 14, 2014; 20(6): 1554-1564
Published online Feb 14, 2014. doi: 10.3748/wjg.v20.i6.1554
Published online Feb 14, 2014. doi: 10.3748/wjg.v20.i6.1554
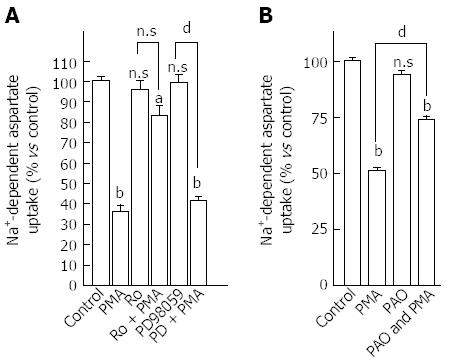
Figure 3 Mechanism pathways modulated by protein kinase C to regulate aspartate uptake capacity in HepG2 cells.
A: Involvement of protein kinase C (PKC) pathway in the modulation of EAAT2 activity in HepG2 cells; Cells were pretreated with 1 μmol/L Ro-31-8220 or vehicle for 15 min. Thereafter, phorbol 12-myristate 13-acetate (PMA) (500 nmol/L) was added and the uptake assay was performed after 15 min. Data shown are means ± SE of three independent experiments performed in triplicate. PMA significantly inhibited D-[3H]-aspartate uptake, Ro-31-8220 had a non-intrinsic effect on aspartate uptake, and completely blocked the effect of PMA on aspartate uptake (Ro-31-8220 vs PMA plus Ro-31-8220 not significantly different). PD09859, a selective inhibitor of the MAPK pathway, failed to prevent the PMA-decrease in aspartate uptake; B: Effect of L-aspartate and phenylarsine oxide (PAO) on basal and PMA-decreased aspartate uptake in HepG2 cells. Cells were pretreated with PAO (10 μmol/L) or vehicle for 15 min. Thereafter, PMA (500 nmol/L) was added and the uptake assay was performed after 15 min. Results are expressed as percent of untreated cells and correspond to means ± SE of at least three independent experiments performed in triplicate. Statistical analysis was performed by One-way ANOVA followed by the Tukey’s test for multiple comparisons. aP < 0.05; bP < 0.001 vs the corresponding untreated control cells; and dP < 0.001 denotes a difference between PMA-treated cells with or without the pharmacological agent.
- Citation: Najimi M, Stéphenne X, Sempoux C, Sokal E. Regulation of hepatic EAAT-2 glutamate transporter expression in human liver cholestasis. World J Gastroenterol 2014; 20(6): 1554-1564
- URL: https://www.wjgnet.com/1007-9327/full/v20/i6/1554.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i6.1554