Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18346
Revised: May 30, 2014
Accepted: July 16, 2014
Published online: December 28, 2014
Processing time: 273 Days and 10.6 Hours
AIM: To investigate the expression of zinc finger protein 139 (ZNF139) in gastric cancer (GC), and to analyze its clinical significance.
METHODS: A total of 108 patients who were diagnosed with GC and underwent surgery between January 2005 and March 2007 were enrolled in this study. Gastric tumor specimens and paired tumor-adjacent tissues were collected and paraffin-embedded, and the clinicopathologic characteristics and prognosis were recorded. The expression of ZNF139, Bcl-2, Bax, and caspase-3 were determined by immunohistochemistry, and apoptosis was assessed by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling. SPSS 13.0 software was used for data processing and analyses, and significance was determined at P < 0.05.
RESULTS: The expression of ZNF139 was stronger in tumors than in tumor-adjacent tissues (66.67% vs 44.44%; P < 0.01). Overexpression of ZNF139 correlated with tumor differentiation, invasion depth, clinical stage, lymphatic metastasis, and blood vessel invasion (all Ps < 0.05). Patients with overexpression of ZNF139 had a poorer prognosis (P < 0.01), and overexpression of ZNF139 was an independent factor for the prognosis of GC patients by a Cox survival analysis (P = 0.02). A negative relationship between ZNF139 and the apoptosis index was observed (r = -0.686; P < 0.01). The expression of Bcl-2 in GC was stronger than in tumor-adjacent tissues (66.67% vs 41.67%), whereas the expression levels of Bax and caspase-3 were lower in primary tumors (54.63% and 47.22%, respectively) than in tumor-adjacent tissues (73.15% and 73.15%, respectively) (all Ps < 0.05). The expression of ZNF139 negatively correlated with caspase-3 (r = -0.370; P < 0.01). The expressions of Bcl-2 and Bax were also negatively correlated (r = -0.231; P = 0.02). The expressions of caspase-3 and Bax protein were positively correlated (r = 0.217; P = 0.024).
CONCLUSION: ZNF139 is related to clinicopathologic characteristics and prognosis of GC. Furthermore, it is overexpressed and involved in apoptosis in GC tissues by regulating caspase-3.
Core tip: We investigated the expression of zinc finger protein 139 (ZNF139) in gastric cancer (GC), and analyzed its clinical significance. The results show that ZNF139 is overexpressed in GC tissues, and is related to clinicopathologic characteristics and prognosis of GC. ZNF139 may be involved in apoptosis in GC tissues by regulating caspase-3.
- Citation: Li Y, Zhao Q, Fan LQ, Wang LL, Tan BB, Leng YL, Liu Y, Wang D. Zinc finger protein 139 expression in gastric cancer and its clinical significance. World J Gastroenterol 2014; 20(48): 18346-18353
- URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18346.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18346
Gastric cancer (GC) is one of the most common malignancies worldwide. It is particularly prevalent in China, South Korea and Japan. Although the incidence and mortality of GC have decreased over the last several years, GC is still a leading cause of mortality in China. The development of GC is complex and involves multi-factorial, multi-targeted and multi-step processes and the mechanism has not yet been totally elucidated[1-4]. The identification of novel GC related genes is of great significance in the pathogenesis and in determining tumor markers and prognostic factors. Zinc finger protein 139 (ZNF139) is a member of the transcription factor ZNF family. It was reported that the expression of ZNF139 was increased in GC tissues[5]. In a previous study, we also found that ZNF139 was closely related to the differentiation of GC cells[6]. These results indicate that ZNF139 may be involved in the carcinogenesis and development of GC. However, to date, there are no systematic reports on the relationship between ZNF139 and GC.
The aim of this study was to investigate the relationship between ZNF139 and GC. We determined the expression of ZNF139 in GC and tumor-adjacent tissues in 108 patients, and analyzed the relationships between ZNF139 and clinicopathologic features and patient prognosis. In addition, as apoptosis plays an important role in the development of GC[7-10], we analyzed the relationship between ZNF139 and the apoptosis index (AI), as well as with expression of the apoptosis-related proteins Bcl-2, Bax and caspase-3. The involvement of ZNF139 in GC progression via regulation of apoptosis was explored.
A total of 108 patients with GC admitted to The Fourth Hospital of Hebei Medical University between January 2005 and March 2007, including 79 males and 29 females, aged between 21 and 86 years (median age 61 years) were enrolled. All the patients underwent surgical treatment, and the clinical data as well as follow-up results were available. The diagnosis of GC was confirmed in all cases by surgery and pathologic examination.
Tumor and adjacent normal mucosa tissue samples (1.0 cm × 1.0 cm × 0.5 cm) were collected, fixed with 10% neutral formalin, embedded in paraffin and then cut serially into 4-μm-thick sections.
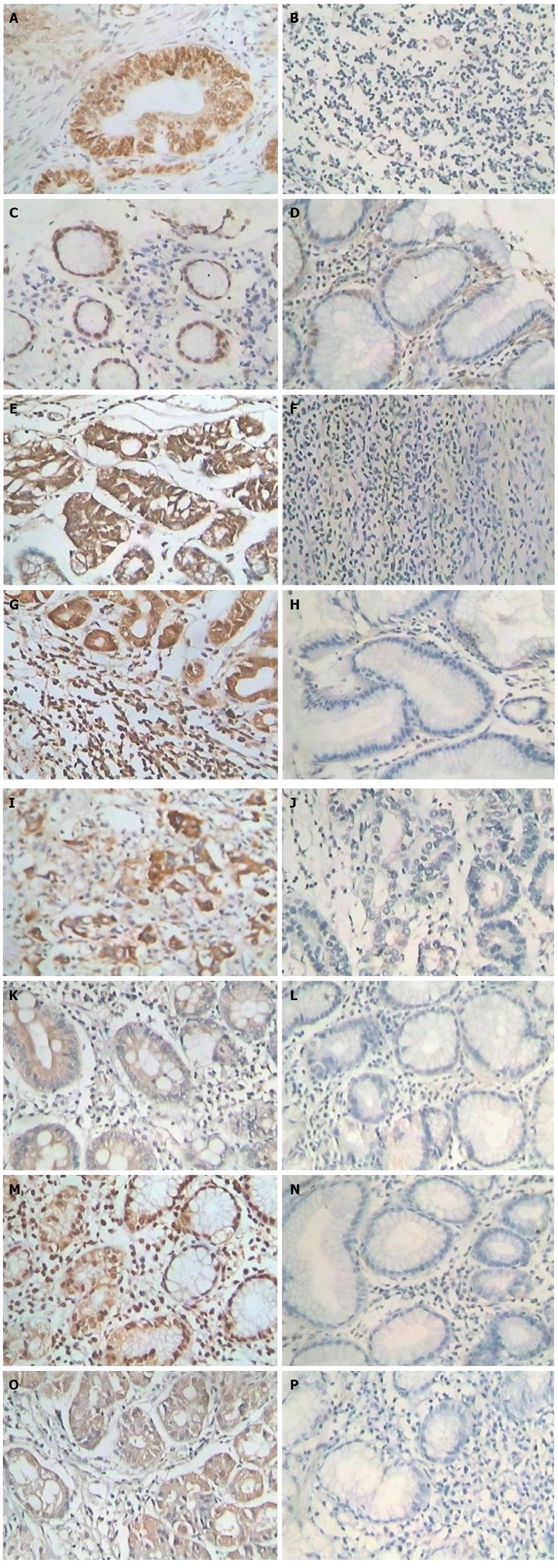
After antigen retrieval, the streptavidin-perosidase (SP) two-step immunohistochemical method was used to detect the expression of ZNF139, Bcl-2, Bax and caspase-3 in GC tissues and tumor-adjacent tissues, following the kit instructions. Rabbit anti-human ZNF-139 polyclonal antibody was purchased from Sigma-Aldrich (St. Louis, MO, United States), and rabbit anti-human Bcl-2, Bax and caspase-3 polyclonal antibodies were purchased from Santa Cruz Inc. (Dallas, TX, United States). The working concentration of the antibodies was 1:100. ZNF139 was positive if the cell nucleus and/or cytoplasm showed brown particles; Bcl-2, Bax and caspase-3 were positive if brown granules appeared in the cytoplasm. Five visual fields were randomly observed under a light microscope at 400 × magnification, and 100 cells were counted in each field. A secondary scoring method was used. First, the sections were scored based on the staining intensity: 0 for colorless, 1 for pale yellow, 2 for brownish yellow and 3 for tan; then positive cells were scored by percentage: 0 for < 25% positive cells, 1 for between 25% and 50% positive cells, 2 for between 51 and 75% positive cells, and 3 for > 75% positive cells. The sum of staining intensity and the percentage of positive cells was regarded as the expression level, with 0 as negative (-), 1-2 as weakly positive (+), 3-4 as positive (++), and 5-6 as strongly positive (+++).
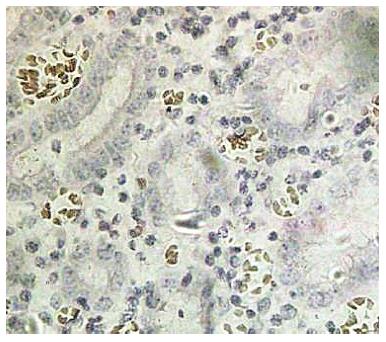
The terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) kit was obtained from Jiangsu Biyuntian Co. (China) and the assay was performed according to the kit instructions. Apoptosis was present if the nuclei underwent pyknosis, shrank to a round or oval shape and were brown or tan, and crescent-shaped chromosomes were observed along the nuclear membrane. Five visual fields at 400 × magnification were examined under a light microscope, and the mean percentage of apoptotic cells from 100 cells counted in each field was calculated and scored as follows: AI < 5% (-), 5%-10% (+), 10%-15% (++) and ≥ 15% (+++).
The χ2 and Wilcoxon signed rank tests and Spearman’s correlation were used to analyze the data. In the analysis of prognosis, the Kaplan-Meier method was employed to calculate survival rate, and the Cox proportional hazards regression model was used for multivariate analysis. All data were processed using SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, United States). A P < 0.05 was considered statistically significant.
ZNF139 was mainly expressed in the cell nucleus and cytoplasm. Staining of positive cells in the area with tumor necrosis was strong, and a general diffuse distribution was observed (Figure 1). The positive expression rate of ZNF139 in GC tissue was 66.67% (72/108), which was significantly higher than in tumor-adjacent tissues (44.44%; 48/108) (P = 0.002). The positive expression rate of Bcl-2 protein in GC tissues was 66.67% (72/108), which was significantly higher than in tumor-adjacent tissues (41.67%; 51/108) (P < 0.001). The positive expression rate of Bax in GC tissues was 54.63% (59/108), which was significantly lower than in tumor-adjacent tissues (73.15%; 79/108) (P = 0.007). The positive expression rate of caspase-3 protein in GC tissue was 47.22% (51/108), which was significantly lower than in tumor-adjacent tissues (73.15%; 79/108) (P < 0.001).
There were no correlations between the expression of ZNF139 and gender or age (both Ps > 0.05). The expression of ZNF139 was correlated with the degree of histologic differentiation of the tumor, invasion depth, clinical stage, lymphatic metastasis, and blood vessel invasion; ZNF139 expression was increased when the degree of tumor differentiation was reduced (P = 0.039). The positive expression rate of ZNF139 in GC stage I-II tissues was 48.84% (21/43), which was significantly lower than in stage III (78.46%, 51/65) (P = 0.002). ZNF139 expression in patients with tumor invasion that was limited to stages T1/T2 (8/20) was significantly lower than in patients with tumor invasion of stages T3/T4 (72.73%; 64/88) (P = 0.008). The positive expression rate of ZNF139 in GC patients without lymph node metastasis was significantly lower than in the patients with lymph node metastasis (P = 0.005) (Table 1).
| Clinical feature | Zinc finger protein 139 | P value | |
| Positive (n) | Negative (n) | ||
| Sex | 0.655 | ||
| Male | 53 | 25 | |
| Female | 19 | 11 | |
| Age (yr) | 0.539 | ||
| ≥ 60 | 43 | 19 | |
| < 60 | 29 | 17 | |
| Tumor differentiation | 0.039 | ||
| High/moderate | 39 | 27 | |
| Poor/undifferentiated | 33 | 9 | |
| Depth of invasion | 0.008 | ||
| T1/T2 | 8 | 12 | |
| T3/T4 | 64 | 24 | |
| Lymphatic metastasis | 0.005 | ||
| Positive | 53 | 16 | |
| Negative | 19 | 20 | |
| Nerve or blood vessel invasion | 0.033 | ||
| Invaded | 29 | 7 | |
| Not invaded | 43 | 29 | |
| Tumor-node-metastasis stage | 0.002 | ||
| I-II | 21 | 22 | |
| III | 51 | 14 | |
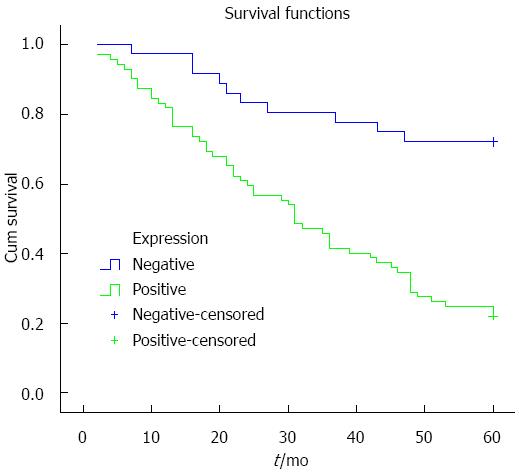
Kaplan-Meier analysis was used to analyze the relationship between ZNF139 expression and prognosis, and the survival curve is shown in Figure 2. Survival rate in patients with positive expression of ZNF139 was lower than in patients with negative expression (P < 0.001). To determine the independent risk factors affecting the prognosis of GC, Cox risk regression analysis was performed using eight indicators as follows: ZNF139 expression, gender, age, degree of tumor differentiation, clinical stage, depth of tumor invasion, lymph node metastasis, and neurovascular invasion. The results show that positive ZNF139 expression, lymph node metastasis, neurovascular invasion and clinical stage are independent risk factors affecting the prognosis of patients with GC (all Ps < 0.05) (Table 2).
| B | SE | Wald | df | P value | Exp(B) | 95%CI for Exp(B) | ||
| Lower | Upper | |||||||
| ZNF139 expression | 0.822 | 0.353 | 5.414 | 1 | 0.02 | 2.276 | 1.138 | 4.551 |
| Lymphatic metastasis | 1.396 | 0.645 | 4.679 | 1 | 0.031 | 4.038 | 1.14 | 14.301 |
| Neurovascular invasion | 0.573 | 0.257 | 4.965 | 1 | 0.026 | 1.774 | 1.072 | 2.939 |
| TNM stage | 1.155 | 0.554 | 4.351 | 1 | 0.037 | 3.175 | 1.072 | 9.401 |
Spearman correlation analysis was used to evaluate the AI and ZNF139 expression in GC, and the results show that the AI is negatively correlated with ZNF139 expression (r = -0.686; P < 0.001) (Figure 3).
Spearman correlation analysis show that ZNF139 protein expression is negatively correlated with caspase-3 expression (r = -0.370; P < 0.001), and not correlated with Bcl-2 or Bax. The expressions of Bcl-2 and Bax protein were negatively correlated (r = -0.231; P = 0.016). In addition, the expressions of caspase-3 and Bax protein were positively correlated (r = 0.217; P = 0.024), and caspase-3 showed no significant correlation with Bcl-2 protein.
The C terminus of proteins in the zinc finger family contains a C2H2 zinc finger motif that can be specifically bound to the regulatory region of target genes or proteins; SCAN and KRAB domains contained in the N terminus can regulate target genes or protein activity by combining the auxiliary effectors[11-14]. Several studies have recently found that many members of the zinc finger protein family are involved in carcinogenesis and the development of tumors. Therefore, research on the transcriptional regulatory function of zinc finger proteins has attracted more attention and has become a research hot spot[15-17]. The ZNF139 gene, located on chromosome 7q21.3-q22.1, was discovered in 1995 and might be involved in gene rearrangement in malignancies in the hematologic system, and absence of its expression is closely related to congenital split-hand/split-foot malformation[11,18]. van Dekken et al[5] found that ZNF139 expression was strongly positive in adenocarcinoma tissues at the gastroesophageal junction, and the expression was also high in normal gastric mucosa of adjacent cancer tissues at proliferation, which suggest that it may possibly participate in the genesis of GC through cell cycle regulation. In our previous study, we found that expression of ZNF139 was closely related to GC, suggesting that ZNF139 might be involved in gastric carcinogenesis and development[6]. Another previous study showed that ZNF139 was involved in GC multidrug resistance by simultaneously promoting the expression of MDR1/P-gp, MRP1 and Bcl-2 and inhibiting Bax[19]. The present study shows that ZNF139 expression in GC tissues is significantly higher than in tumor-adjacent tissues, which indicates that ZNF139 may be involved in gastric carcinogenesis and development. Thus, further studies on ZNF139 may help to elucidate the pathogenesis of GC.
In present study, we analyzed the relationship between the expression of ZNF139 in GC tissues and clinicopathologic features in patients. It was found that ZNF139 protein expression in GC was closely related to the degree of histologic differentiation, clinical stage, depth of invasion, lymph node metastasis, and neurovascular invasion. That is, the lower the degree of tumor differentiation, the later the clinical stage, the deeper the invasion. In addition, lymph node metastases and neurovascular invasion contributed to higher ZNF139 expression, indicating that ZNF139 is related to the development of GC and the degree of malignancy; this gene was further enhanced during tumor progression and might contribute to the progress of GC. The prognostic analysis showed that the five-year survival rate in patients with positive ZNF139 expression was significantly lower than in patients with negative expression, and ZNF139 was an independent risk factor affecting the prognosis of GC patients. These results show that ZNF139 may play an important role as an oncogene in the development of GC, and may contribute to the diagnosis and treatment of GC as a new tumor marker and a predictor of prognosis.
To determine the mechanisms of ZNF139’s involvement in GC progression, we analyzed the relationship between ZNF139 and tumor cell apoptosis. Apoptosis of tumor cells can be assessed using the TUNEL assay to calculate the AI[20,21], a method used in the present study to examine the relationship between ZNF139 expression and apoptosis. The results show that ZNF139 protein is negatively correlated with the AI, suggesting that positive ZNF139 expression may inhibit tumor cell apoptosis. Further investigation demonstrated that ZNF139 expression is negatively correlated with the apoptosis-related protein caspase-3, but not with Bcl-2 or Bax. Bcl-2 and Bax are important genes in the regulation of tumor cell apoptosis through a mitochondrial pathway, whereas activation of caspase-3 can directly promote apoptosis[22-27]. These results suggest that ZNF139, as a nuclear transcription factor, may directly regulate the expression and inhibit activity of caspase-3, thus prevent apoptosis and contribute to the progression of GC. However, the exact mechanism requires further study.
This study shows that ZNF139 is upregulated in GC and related to some of the clinicopathologic features, and thus may be involved in gastric carcinogenesis and progression. ZNF139 may be used as a prognostic factor as GC patients with strong ZNF139 expression had a poor prognosis. These effects of ZNF139 may be related to the regulation of caspase-3 expression, and thus participate in the regulation of apoptosis in GC. However, identification of the detailed mechanisms of ZNF139 in GC cells requires further investigation. Our findings suggest that ZNF139 may play an important role as an oncogene in the development of GC and further research on ZNF139 may lead to clarification of the development of GC and thus treatment.
Previous research showed that zinc finger protein 139 (ZNF139) was overexpressed in gastric cancer (GC) cells, which was related to the differentiation of GC. However, there is no research on the relationship between ZNF139 and the clinicopathologic characteristics and prognosis of GC.
A number of oncogenes are involved in the carcinogenesis and development of GC, and, to date, researchers have not identified an oncogene that could be a prognostic factor. In the present study, the clinical value of ZNF139 in GC was explored.
There are many reports on the effect of new genes in GC. In the present study, authors found that ZNF139 was closely related to the clinicopathologic characteristics and prognosis of GC, thus, ZNF139 may play an important role in GC.
ZNF139 can be used as a tumor marker and prognostic factor to evaluate the prognosis of patients with GC. In addition, ZNF139 may regulate apoptosis of GC cells, and the ZNF139 gene may be a target in gene therapy of GC.
A zinc finger is a small structural protein motif that is characterized by the coordination of one or more zinc ions in order to stabilize the fold. Originally coined to describe the finger-like appearance of a hypothesized structure from Xenopus laevis transcription factor IIIA, the zinc finger has been found in a wide variety of different protein structures. Immunohistochemistry refers to the process of detecting antigens (i.e., proteins) in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biologic tissues. The apoptosis index describes the proportion of apoptotic cells in the total cell population.
In this study, the relationships between ZNF139 and the clinicopathologic characteristics and prognosis of GC were investigated. The authors propose that ZNF139 may regulate apoptosis of GC cells by regulating caspase-3. It is a new discovery regarding the mechanism of carcinogenesis and development of GC. Tests evaluating ZNF139 may be applied to evaluate the prognosis of GC patients.
P- Reviewer: Pantakani DVK, Wu S, Yamashita M S- Editor: Gou SX L- Editor: AmEditor E- Editor: Ma S
| 1. | Chen Z, Huang Y, Shen X, Guo J, Zhu G, Dralle H, Hoang-Vu C. Short hairpin RNA targeting autotaxin reduces human gastric carcinoma AGS cell proliferative, migratory and invasive capabilities in vitro and causes tumor regression in vivo. Oncol Rep. 2013;29:1087-1093. [PubMed] |
| 2. | Xu S, Zhou Y, Du WD, Chen G, Zhou FS, Schneider M, Ma XL, Xu HY, Zhang XJ. Association of the variant rs2243421 of human DOC-2/DAB2 interactive protein gene (hDAB2IP) with gastric cancer in the Chinese Han population. Gene. 2013;515:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Ma GF, Chen SY, Sun ZR, Miao Q, Liu YM, Zeng XQ, Luo TC, Ma LL, Lian JJ, Song DL. FoxP3 inhibits proliferation and induces apoptosis of gastric cancer cells by activating the apoptotic signaling pathway. Biochem Biophys Res Commun. 2013;430:804-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Saeki N, Ono H, Sakamoto H, Yoshida T. Genetic factors related to gastric cancer susceptibility identified using a genome-wide association study. Cancer Sci. 2013;104:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | van Dekken H, Tilanus HW, Hop WC, Dinjens WN, Wink JC, Vissers KJ, van Marion R. Array comparative genomic hybridization, expression array, and protein analysis of critical regions on chromosome arms 1q, 7q, and 8p in adenocarcinomas of the gastroesophageal junction. Cancer Genet Cytogenet. 2009;189:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Li Y, Tan BB, Fan LQ, Zhao Q, Song ZC, Wang D. [Proteomic identification and comparison of differentiation-related proteins in gastric carcinoma cell lines]. Zhonghua Zhong Liu Zazhi. 2010;32:179-184. [PubMed] |
| 7. | Sun KW, Ma YY, Guan TP, Xia YJ, Shao CM, Chen LG, Ren YJ, Yao HB, Yang Q, He XJ. Oridonin induces apoptosis in gastric cancer through Apaf-1, cytochrome c and caspase-3 signaling pathway. World J Gastroenterol. 2012;18:7166-7174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Song NM, Jun S, Zang DY, Kim SG, Park HR, Kang D. Differential susceptibility of gastric cancer cells to TRAIL-induced apoptosis. Oncol Rep. 2013;29:1224-1230. [PubMed] |
| 9. | Hayakawa Y, Hirata Y, Sakitani K, Nakagawa H, Nakata W, Kinoshita H, Takahashi R, Takeda K, Ichijo H, Maeda S. Apoptosis signal-regulating kinase-1 inhibitor as a potent therapeutic drug for the treatment of gastric cancer. Cancer Sci. 2012;103:2181-2185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Kundu J, Wahab SM, Kundu JK, Choi YL, Erkin OC, Lee HS, Park SG, Shin YK. Tob1 induces apoptosis and inhibits proliferation, migration and invasion of gastric cancer cells by activating Smad4 and inhibiting β-catenin signaling. Int J Oncol. 2012;41:839-848. [PubMed] |
| 11. | Edelstein LC, Collins T. The SCAN domain family of zinc finger transcription factors. Gene. 2005;359:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Wei J, Yuan Y, Jin C, Chen H, Leng L, He F, Wang J. The ubiquitin ligase TRAF6 negatively regulates the JAK-STAT signaling pathway by binding to STAT3 and mediating its ubiquitination. PLoS One. 2012;7:e49567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Tsai SJ, Hwang JM, Hsieh SC, Ying TH, Hsieh YH. Overexpression of myeloid zinc finger 1 suppresses matrix metalloproteinase-2 expression and reduces invasiveness of SiHa human cervical cancer cells. Biochem Biophys Res Commun. 2012;425:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Liu G, Jiang S, Wang C, Jiang W, Liu Z, Liu C, Saiyin H, Yang X, Shen S, Jiang D. Zinc finger transcription factor 191, directly binding to β-catenin promoter, promotes cell proliferation of hepatocellular carcinoma. Hepatology. 2012;55:1830-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Miyake N, Katoh O, Hirata S, Kimura S, Watanabe H, Yajin K. Expression of the Krüppel-type zinc finger gene, ZK7, in head and neck squamous cell carcinoma and normal mucosa. Cancer Lett. 2002;185:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Li Y, Liang Q, Wen YQ, Chen LL, Wang LT, Liu YL, Luo CQ, Liang HZ, Li MT, Li Z. Comparative proteomics analysis of human osteosarcomas and benign tumor of bone. Cancer Genet Cytogenet. 2010;198:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Yang L, Hamilton SR, Sood A, Kuwai T, Ellis L, Sanguino A, Lopez-Berestein G, Boyd DD. The previously undescribed ZKSCAN3 (ZNF306) is a novel “driver” of colorectal cancer progression. Cancer Res. 2008;68:4321-4330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Tommerup N, Vissing H. Isolation and fine mapping of 16 novel human zinc finger-encoding cDNAs identify putative candidate genes for developmental and malignant disorders. Genomics. 1995;27:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Li Y, Tan BB, Zhao Q, Fan LQ, Liu Y, Wang D. Regulatory mechanism of ZNF139 in multi-drug resistance of gastric cancer cells. Mol Biol Rep. 2014;41:3603-3610. [PubMed] |
| 20. | Wang G, Han B, Zhou H, Wu L, Wang Y, Jia G, Lv J, Cheng Z, Pan S, Liu J. Inhibition of hydrogen sulfide synthesis provides protection for severe acute pancreatitis rats via apoptosis pathway. Apoptosis. 2013;18:28-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Bernardo PS, Reis FR, Maia RC. Imatinib increases apoptosis index through modulation of survivin subcellular localization in the blast phase of CML cells. Leuk Res. 2012;36:1510-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Wang Y, Wang X, Zhao H, Liang B, Du Q. Clusterin confers resistance to TNF-alpha-induced apoptosis in breast cancer cells through NF-kappaB activation and Bcl-2 overexpression. J Chemother. 2012;24:348-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Yoon O, Roh J. Downregulation of KLF4 and the Bcl-2/Bax ratio in advanced epithelial ovarian cancer. Oncol Lett. 2012;4:1033-1036. [PubMed] |
| 24. | Jeong SH, Han JH, Kim JH, Ahn MS, Hwang YH, Lee HW, Kang SY, Park JS, Choi JH, Lee KJ. Bax predicts outcome in gastric cancer patients treated with 5-fluorouracil, leucovorin, and oxaliplatin palliative chemotherapy. Dig Dis Sci. 2011;56:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Park JS, Park JH, Lee S, Joo YE, Jung YD. Small interfering RNA targeting of Recepteur d’Origine Nantais induces apoptosis via modulation of nuclear factor-kappaB and Bcl-2 family in gastric cancer cells. Oncol Rep. 2010;24:709-714. [PubMed] |
| 26. | Chen J, Yang B, Zhang S, Ling Y, Ye J, Jia Z, Cao J. Antitumor potential of SLPI promoter controlled recombinant caspase-3 expression in laryngeal carcinoma. Cancer Gene Ther. 2012;19:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Zhang D, Liu J, Wang Y, Chen J, Chen T. shRNA-mediated silencing of Gli2 gene inhibits proliferation and sensitizes human hepatocellular carcinoma cells towards TRAIL-induced apoptosis. J Cell Biochem. 2011;112:3140-3150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |