Published online Dec 21, 2014. doi: 10.3748/wjg.v20.i47.17976
Revised: July 5, 2014
Accepted: August 13, 2014
Published online: December 21, 2014
Processing time: 243 Days and 6.2 Hours
AIM: To compare XELOX and FOLFOX4 as colon cancer adjuvant chemotherapy based on MOSAIC and No. 16968 trails from Chinese cost-effectiveness perspective.
METHODS: A decision-analytic Markov model was developed to compare the FOLFOX4 and XELOX regimens based MOSAIC and No. 16968 trial. Five states were included in our Markov model: well (state 1), minor toxicity (state 2), major toxicity (state 3), quitting adjuvant chemotherapy (state 4), and death due to adjuvant chemotherapy (state 5). Transitions among the 5 states were assumed to be Markovian. Costs were calculated from the perspective of the Chinese health-care payer. The utility data were taken from published studies. Sensitivity analyses were used to explore the impact of uncertainty factors in this cost-effectiveness analysis.
RESULTS: Total direct costs of FOLFOX4 and XELOX per patient were $19884.96 ± 4280.30 and $18113.25 ± 3122.20, respectively. The total fees related to adverse events per patient during the entire treatment were $204.75 ± 16.80 for the XELOX group, and $873.72 ± 27.60 for the FOLFOX4 group, and the costs for travel and absenteeism per patient were $18495.00 for the XELOX group and $21,352.68 for the FOLFOX4 group. The base-case analysis showed that FOLFOX4 was estimated to produce an additional 0.06 in quality adjusted life years (QALYs) at an additional cost of $3950.47 when compared to the XELOX regimen over the model time horizon. The cost per QALY gained was $8047.30 in the XELOX group, which was $900.98 less than in the FOLFOX4 group ($8948.28). The one way sensitivity analysis demonstrated that the utility for the well state and minor toxicity state greatly influenced the incremental cost-effectiveness ratio of FOLFOX4.
CONCLUSION: In term of cost-comparison, XELOX is expected to dominate FOLFOX4 regimes; Therefore, XELOX provides a more cost-effective adjuvant chemotherapy for colon cancer patients in China.
Core tip: Notably, patients with stage III colon cancer are recommended to receive either XELOX or FOLFOX4 as adjuvant therapy. However, there has not been a cost-effectiveness analysis of these two regimens. This study compared XELOX and FOLFOX4 as adjuvant chemotherapy for patients with colon cancer based on the MOSAIC and No. 16968 trails from a Chinese cost-effectiveness perspective. Our results demonstrated that XELOX was a more cost-effective treatment for adjuvant chemotherapy of colon cancer in China.
- Citation: Wen F, Yao K, Du ZD, He XF, Zhang PF, Tang RL, Li Q. Cost-effectiveness analysis of colon cancer treatments from MOSIAC and No. 16968 trials. World J Gastroenterol 2014; 20(47): 17976-17984
- URL: https://www.wjgnet.com/1007-9327/full/v20/i47/17976.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i47.17976
Colorectal cancer ranks third among the leading causes of cancer-associated death in both males and females of all ages worldwide after lung and breast cancer[1]. Nearly 70% colorectal cancers are located in the colon, and approximately 26% are in the stage III when they were first diagnosed. What is even worse, it is estimated that about 50%-60% of patients will suffer a recurrence after radical resection[2].
Recently, many results of large clinical randomized controlled studies have showed that adjuvant chemotherapy after standard surgery and radiation for early stage colon cancer takes an active part in decreasing the risk of recurrence and extend overall survival[3]. An early pooled analysis has shown the efficacy of 5-fluorouracil/leucovorin (FU/LV) treatment with a significant reduction of the recurrence rate by 35%, and death rate by 22%, when compared with no treatment in patients with a curative resection of colon cancer[4]. Additionally, FU/LV has been shown to have the best efficacy among chemotherapy regimens, such as the FU and levamisole regimens[5].This has led to FU/LV being the standard adjuvant chemotherapy for colon cancer until, that is, the MOSAIC trial found that FOLFOX4 (5-Fu/LV + OX) significantly increased disease-free survival (DFS) and overall survival (OS) when used as adjuvant treatment for stage II or III colon cancer[6,7].
Subsequently in 2011, the No. 16968 trial showed that compared with 5-FU/LV, XELOX led to significantly better 3-year DFS (66.5% vs 70.9%) and higher 5-year OS rate(74.2% vs 77.6%) in patients with stage III colon cancer, respectively[8]. Therefore, based on the clinical practice guidelines of the 2011 National Comprehensive Cancer Network (NCCN), FOLFOX4 and XELOX were standard adjuvant treatments of colon cancer[9].
Several studies have consistently reported that compared with FU/LV, FOLFOX is a cost-effective adjuvant chemotherapy for patents with stage II and III colon cancer from the perspectives of United States, Canada and Japan[10-12]. Shiroiwa et al[13] suggested that XELOX, as first-line and second-line chemotherapy, was superior to FOLFOX4 in term of cost and effectiveness. Previously, we have indicated that XELOX is expect to outperform the FOLFOX4 regimen in China based on a simple cost analysis[14]. However, to the best of our knowledge, we do not know which regimen is more cost-effective. Hence, in this study we determined to find a more affordable adjuvant chemotherapy option between XELOX and FOLFOX4 for colon cancer patients based on data obtained from the MOSAIC and No. 16968 trials from a Chinese cost-effectiveness perspective.
A Markov model was constructed to estimate the incremental cost-effectiveness of FOLFOX4 compared with XELOX as adjuvant treatments for patients who had stage II or III colon cancer in a Chinese health-care setting based on data obtained from the MOSAIC trial and No. 16968 trial. Our analysis was performed from a third-party health-care payer’s perspective, which included both direct medical costs and indirect costs. Quality adjusted life year (QALY) gained, and an estimate of overall costs, were used to evaluate the incremental cost-effectiveness ratio (ICER) of both treatments.
According to the No. 16968 trial, the XELOX regimen was a 3-wk cycle treatment for 8 cycles, including a 2-h intravenous infusion of oxaliplatin (130 mg/m2) on day 1 and oral capecitabine (1000 mg/m2) twice a day from day 1 to day 14[8]. FOLFOX4 was consisted of 2-h intravenous infusions of oxaliplatin(85 mg/m2) and LV 200 mg/m2 on day 1, followed by a bolus of 400 mg/m2 of 5-FU and a 22-h infusion of 5-FU 600 mg/m2 lasting 22 h every 14 d, for 12 cycles[6].
In our base-case analysis, all expenses were separated into three categories: direct costs of chemotherapy (fees for anti-cancer drugs, hospitalization, venous access, experimental tests and pre-chemotherapy drugs, such as antiemetics and hepatoprotective drugs), costs of treatments related to adverse effects (for example, recombinant human granulocyte/macrophage colony-stimulating factor), and costs from a societal perspective (i.e., travel fees and absenteeism cost). Direct chemotherapy costs and adverse effects related costs were analyzed from the perspective of healthcare providers, and travel costs and absenteeism costs were from societal perspective. Travel costs were based on taxi fare per kilometer in Sichuan, China. According to the median monthly salary in Sichuan, China (patient’s salaries were not recorded in the medical records), equal costs were estimated for patients’ hospital time. All the costs were translated into United States dollars. Other unrelated care fees were not calculated in the study.
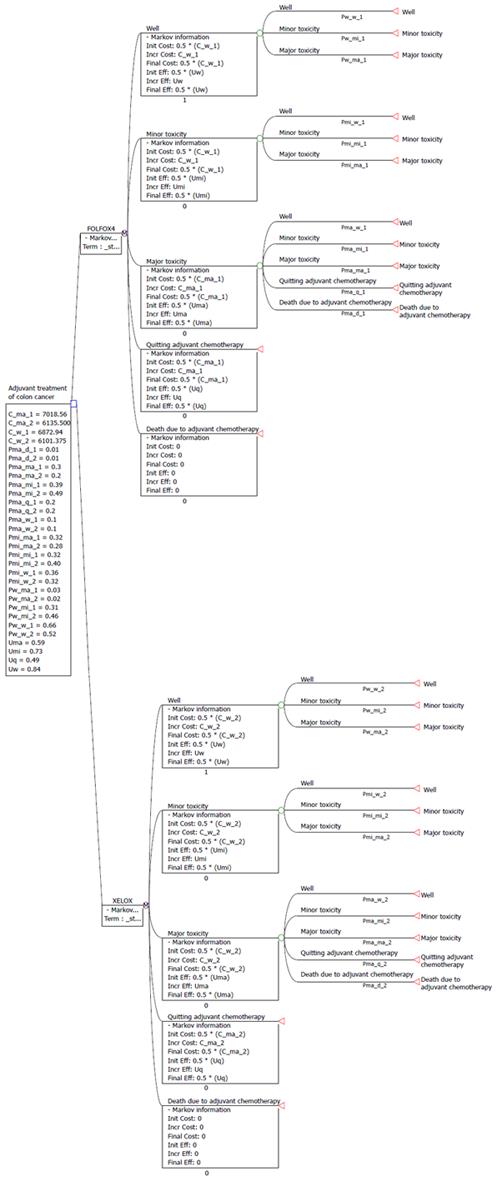
Referring to the cost-effectiveness analysis of adjuvant chemotherapy for colon cancer patients, 5 states were included in our Markov model (Figure 1) and states 1-5 were listed orderly as follows: well, minor toxicity, major toxicity, quitting adjuvant chemotherapy, and death due to adjuvant chemotherapy[15]. Transitions among the 5 states were assumed to be Markovian.
The first three states represented the toxicity reaction of patients received treatments according to the Common Toxicity Criteria of the National Cancer Institute, version 3, and the records of adverse events of the MOSAIC trial and No. 16968 trial, where ‘‘well’’ represented grade 0 toxicity, ‘‘minor toxicity’’ represented grade 1 and 2 toxicity, and ‘‘major toxicity’’ represented grade 3 and 4 toxicity, and severe or life-threatening adverse effects[16,17]. The cycle length of the Markov model was set to 1 mo for a total of 6 mo-specifically, the transitions occurred once a month for 6 times. At the start of the adjuvant chemotherapy, all patients began in the “well” state (free of cancer).
Pw-w, Pw-mi, Pw-ma, Pmi-w, Pmi-mi, Pmi-ma, Pma-w, Pma-mi, Pma-ma, Pma-q, and Pma-d were applied to denote the probabilities of transition of the model, in which suffix w represented the first state(well state), mi represented state of minor toxicity, ma represented state of major toxicity, q represented state of quitting the adjuvant chemotherapy, and d represented death state due to the adjuvant chemotherapy. Pw-mi represented the probability of the changing of patient in the well state in the current cycle to the minor toxicity state in the next cycle. Because the incidence of toxicity in our patients was consistent with the incidences found in several other clinical studies, the values for adverse events values reported in the MOSAIC trial and No. 16968 trial for FOLFOX4 and XELOX chemotherapy were applied in this study. A calibration method was used to estimate the exact values[15,18]. The parameter values are listed in Table 1.
| Value | |||
| Parameters | FOLFOX4 | Data source | XELOX |
| Overall toxicity after adjuvant chemotherapy | |||
| Percentage of major toxicity state Pma | 50.9% | 43.0% | Andréand others, 2004 |
| Percentage of minor toxicity state Pmi | 41.1% | 55.0% | Hans-Joachim Schmoll and others, 2007 |
| Percentage of well state Pw | 8.0% | 2.0% | |
| Percentage of death due to adjuvant chemotherapy Pd | 0.5% | 0.6% | |
| Percentage of quitting adjuvant chemotherapy Pq | 25.3% | 22.0% | |
| Probability of well state to well state Pw-w | 0.66 | 0.52 | Mehmet US Ayvaci and others, 2012 |
| Probability of well state to minor toxicity state Pw-mi | 0.31 | 0.46 | |
| Probability of well state to major toxicity state Pw-ma | 0.03 | 0.02 | |
| Probability of minor toxicity state to well state Pmi-w | 0.36 | 0.32 | |
| Probability of minor toxicity state to minor toxicity state Pmi-mi | 0.32 | 0.40 | |
| Probability of minor toxicity state to major toxicity state Pmi-ma | 0.32 | 0.28 | |
| Probability of major toxicity state to well state Pma-w | 0.10 | 0.10 | |
| Probability of major toxicity state to minor toxicity state Pma-mi | 0.39 | 0.49 | |
| Probability of major toxicity state to major toxicity state Pma-ma | 0.30 | 0.20 | |
| Probability of major toxicity state to quitting adjuvant chemotherapy Pma-q | 0.20 | 0.20 | |
| Probability of major toxicity state to death due to adjuvant chemotherapy Pma-d | 0.01 | 0.01 | |
Several related studies were examined to determine the utility values of the 5 states in the adjuvant chemotherapy[15,19]. The utility for well state patients was set to 0.84, which was the same for patients without adjuvant chemotherapy after surgery based on the study by Ramsey et al[19] and van Hout et al[20]. With regard to the utilities of minor toxicity and major toxicity, the means of utilities of patients with moderate or severe adverse events were calculated. The values are shown in Table 2.
| Parameters | Value | Data source |
| Well state | 0.84 | Mehmet US Ayvaci and others, 2012 |
| Minor toxicity state | 0.73 | |
| Major toxicity state | 0.59 | |
| Quitting adjuvant chemotherapy | 0.47 | |
| Death due to adjuvant chemotherapy | 0.00 |
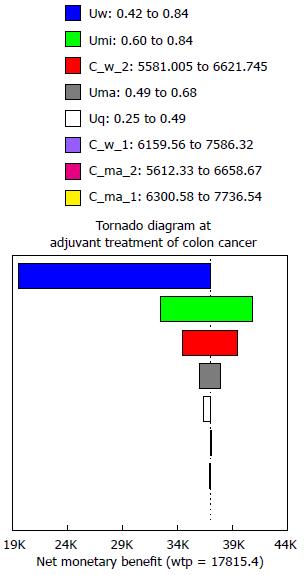
One-way deterministic sensitivity analyses, a tornado diagram and threshold analysis were used to identify key model input parameters that could potentially influence the results over the low/high value, such as direct costs of chemotherapy, adverse related fees, societal costs, and utility scores. Based on the influence of the variables on the incremental net health benefit, a tornado diagram was applied. Willingness to pay was set to $17815.40, triple the per capita GDP of China according to the guidelines of World Health Organization for cost-effectiveness analysis[21,22].
No significant differences were identified for male/female ratio, age, depth of invasion and histology between the FOLFOX4 group and the XELOX group based on the MOSAIC trial and the No. 16968 trial (Table 3).
| FOLFOX4 (n = 1123) | XELOX (n = 944) | |
| Male, % | 56.1 | 54 |
| Age, yr | 61 | 61 |
| Depth of invasion, % | ||
| T1-2 | 4.5 | 11 |
| T3 | 76 | 74 |
| T4 | 19 | 15 |
| TX | 0.5 | < 1 |
| Histology, % | ||
| Differentiated | 83.2 | 81 |
| Poorly differentiated | 12.6 | 15 |
| Unknown | 4.2 | 3 |
According to the cases in our study, the total direct costs of FOLFOX4 were 109.8% as high as those of XELOX per patient, which were $19884.96 ± 4280.30 and $18113.25 ± 3,122.20, respectively. Total fees related to adverse events per patient for the entire treatment were $204.75 ± 16.80 for the XELOX group, and $873.72 ± 27.60 for the FOLFOX4 group. For the costs from a societal perspective, specially, fees for travel and absenteeism, the average travel costs per cycle were set at $7.90 for taxi identically in two groups. Therefore, total travel costs would be $59.25 for XELOX per patient and $85.32 for FOLFOX4. Pay for one day was $15.70 according to the average monthly salary of $478 in Sichuan, China. As a result, the estimated costs for absenteeism for chemotherapy per patient were $508.68 in FOLFOX4 group and $117.75 in XELOX group, respectively. Hence, the total fees for travel and absenteeism per patient were $18495.00 for the XELOX group and $21352.68 for the FOLFOX4 group (Table 4).
| Parameters | FOLFOX4 ($) | XELOX ($) | Data source |
| Direct costs/mo | 3314.16 ± 713.38 | 3018.88 ± 520.37 | Xie et al[14] and others, 2013 |
| Adverse event costs/mo | 145.62 ± 4.6 | 34.13 ± 2.8 | |
| Societal costs/mo | 3558.78 | 3082.5 | |
| Cost for well state (Cw1/Cw2) | 6872.94 ± 713.38 | 6101.38 ± 520.37 | |
| Cost for minor toxicity state (Cmi1/Cmi2) | Cw1 | Cw2 | |
| Cost for major toxicity state (Cma1/Cma2) | 7018.56 ± 717.98 | 6135.50 ± 523.17 | |
| Cost for quitting adjuvant chemotherapy (Cq1/Cq2) | Cma1 | Cma2 |
Based on the data collected above, the cost for the well state was $6101.38 ± 520.37 in the XELOX group, and $6872.94 ± 713.38 in the FOLFOX4 group, which was identical to the minor toxicity state. The cost for the major toxicity state and quitting adjuvant chemotherapy state $6135.50 ± 523.17 in the XELOX group, and $7018.56 ± 717.98 in the FOLFOX4 group.
After running our Markov model for the 5 stages, the cumulative cost and cumulative effect were $30466.45 and 3.79 quality-adjusted life years for the XELOX group, and $34416.92 and 3.85 quality-adjusted life years for the FOLFOX4 group. Though the FOLFOX4 regimen was estimated to produce an additional 0.06 QALYs, the additional cost was significant ($3950.47) compared to the XELOX regimen over the model time horizon. The cost per QALY gained was $8047.30 in the XELOX group, which is $900.98 less than in the FOLFOX4 group ($8948.28). The results showed that XELOX was expected to dominate FOLFOX4 regimens; in other words, XELOX was a more cost-effective adjuvant treatment for colon cancer (Table 5).
| Parameters | FOLFOX4 | XELOX |
| Whole cost | $34416.92 | $30466.45 |
| QALY | 3.85 | 3.79 |
| Cost/effect | $8948.28 | $8047.30 |
| ICER | $15016.33 | - |
| Threshold (/QALY) | $17815.4 | $17815.4 |
The results of the sensitivity analysis for “cost for well state” (C_w_1 for FOLFOX4, C_w_2 for XELOX) and “cost for major toxicity state” (C_ma_1 for FOLFOX4, C_ma_2 for XELOX), and the “utility scores for 5 states” are shown in the tornado diagram (Figure 2). The results of one-way deterministic sensitivity analyses showed that the utility for the well state and the minor toxicity state greatly influenced the ICER of FOLFOX4. When the utility score of the minor toxicity state changed from 0.60 to 0.84, the ICER increased from $30242 per QALY gained to $5205921 per QALY gained, which was a highly significant increase. Among all the cost factors for the 5 states in the two groups, the cost for well state for the XELOX group (C_w_2) played a key role in our analysis, which varied from $5581.01 to $6621.75 and resulted in the ICER decreasing from $107342 per QALY gained to $23724 per QALY gained.
New adjuvant therapies for colon cancer have increased the overall survival, meanwhile patients’ quality of life has been improved. However, a dramatic economic burden was produced with the widespread use of adjuvant treatments[13,21,23,24]. Indeed, the head-to-head comparisons trials of these different therapies are seldom, and a cost-effectiveness evaluation of the standard adjuvant chemotherapies in a health resource-limited setting is of critical importance to address the balance between health care costs and benefits. With indirect comparison and decision analysis modeling techniques, monetary costs and therapeutic efficacies of the adjuvant chemotherapy regimens FOLFOX4 and XELOX for patients with colon cancer were estimated over an adjuvant chemotherapy horizon in term of Chinese healthcare system based on MOSAIC trial and No. 16968 trial.
The XELOX group not only showed advantages in term of costs of hospitalization, and time and travel costs for the patient, but also in the cost of venous access. Based on these cost data, our results showed the whole cost of the XELOX regimen was 86.6% that of the FOLFOX4 regimen for an average patient, even though the gained quality-adjusted life years were approximately the same; 3.79 for XELOX and 3.84 for FOLFOX4. Though the FOLFOX4 regimen was estimated to produce an additional 0.06 QALYs, the additional cost was significant ($3950.47) compared to the XELOX regimen over the model time horizon. However, the cost was $8047.30/QALY in the XELOX group, and $8948.28/QALY in the FOLFOX4 group, which shows that the cost for the FOLFOX4 group was $900.98 greater. The results indicated that XELOX is worth considering as an alternative to the FOLFOX4 regimen and the results were consistent with the cost-effectiveness analysis in metastatic colorectal cancer[13].
In China, no consensus has been reached on the threshold of acceptable cost per QALY saved. A threshold range of £20000 to £30000 is used in the National Institute for Health and Clinical Excellence in the United Kingdom[25], whereas the United States often applies a threshold range of $50000 to $100000. Based on the guidelines of the World Health Organization (WHO) for cost-effectiveness analysis, the willingness to pay $17815.40, which is triple the per capita GDP of China, is an appropriate threshold[21]. Considering all the criteria above, the costs per QALY in the FOLFOX4 group and the XELOX group, as well as the ICER for FOLFOX4, were thought to be acceptable.
In our research, the rates of grade 3/4 adverse events related to FOLFOX4 and XELOX were obtained from the cross-trial comparison of the MOSAIC trial and No. 16968 trial, which showed that the incidences of diarrhea, stomatitis, nausea, vomiting, neurosensory, hand-foot syndrome (HFS), neutropenia and febrile neutropenia were 19% and 12%, 0.6% and 3%, 5% and 5%, 6% and 6%, 11% and 12%, 5% and 2%, 9% and 41%, 0.2% and 2% for FOLFOX4 and XELOX, respectively[7]. The differences were statistically significant for stomatitis, neutropenia and febrile neutropenia, resulting in higher adverse events-associated costs for FOLFOX4 regimen than that for XELOX regimen. Therefore, the cost for the major toxicity state was significantly higher in the FOLFOX4 group. However, from the results of one-way deterministic sensitivity analyses, it was not the cost for the major toxicity state (C_ma_2) but the cost for the well state for the XELOX group (C_w_2) that, which played a key role in our analysis-this varied from $5581.01 to $6621.75 and resulted in a reduction of ICER from $107342 per QALY gained to $23724 per QALY gained. Our results indicated that adverse effect-related costs might not be as important a factor to consider for optimal adjuvant chemotherapy.
To our surprise, the ICER of FOLFOX4 was greatly influenced by the utility scores for the well state and minor toxicity state. When the utility score of the minor toxicity state changed from 0.60 to 0.84, the ICER increased from $30242 per QALY gained to $5205921 per QALY gained. The reason for this may be that status of patients in adjuvant chemotherapy play a key role in estimating the QALY and evaluating the ICER[26].
Our research is partly based on patient-level data which were collected from the MOSAIC trial and the No. 16968 trial, and on the data collected from the West China Hospital, Sichuan University, China, which is the main limitation of our research. The potential heterogeneity of effectiveness and resource consumption between Asians and the international population may be another significant limitation in this research. Additionally, although we have analyzed the costs related to adverse events and societal costs, such as fees for travel and absenteeism, which are seldom included in other related cost-effectiveness analyses. Additionally, supportive care costs were not included in our analysis because that it is difficult to collect the detail from medical records. Finally, as we do not have direct head-to-head comparisons on the effectiveness of FOLFOX4 and XELOX for adjuvant treatment of patients with colon cancer in large randomized controlled trials, more detail should be studied to figure out the further cost-effectiveness of the two regimens.
Notably, it is the first study to compare cost-effectiveness option of adjuvant chemotherapy for colon cancer between FOLFOX4 and XELOX. We found that XELOX could achieve the maximum level of clinical benefit over other adjuvant treatments, which is a more affordable option in China. This result is worthy of consideration for both doctors and patients, and provides decision makers a more comprehensive view of treatment-related cost-effectiveness in clinical practice.
Based on the clinical practice guidelines of the 2011 National Comprehensive Cancer Network, FOLFOX4 and XELOX were standard adjuvant treatments of colon cancer. Several studies have consistently reported that compared with FU/LV, FOLFOX is a cost-effective adjuvant chemotherapy for patents with stage II and III colon cancer. However, which is a more affordable option of adjuvant chemotherapy for colon cancer in the terms of cost-effectiveness analysis is still unknown.
The base-case analysis showed that FOLFOX4 was estimated to produce an additional 0.06 in quality adjusted life years at an additional cost of $3950.47 when compared to the XELOX regimen over the model time horizon. The cost per QALY gained was $8047.30 in the XELOX group, which is $900.98 less than in the FOLFOX4 group ($8948.28). XELOX is expected to dominate FOLFOX4 regimens at a point view of cost-comparison point of view; in other words, XELOX was a more cost-effective treatment for adjuvant chemotherapy for patients with colon cancer in China.
It is the first study to compare cost-effectiveness option of adjuvant chemotherapy for colon cancer between FOLFOX4 and XELOX. The authors found that XELOX could achieve the maximum level of clinical benefit over other adjuvant treatments, while being a more affordable option in China.
This result is worthy of consideration for both doctors and patients, and provides decision makers a more comprehensive view of treatment-related cost-effectiveness in clinical practice.
Five states were included in our Markov model and states 1 to 5 were as follows orderly: well, minor toxicity, major toxicity, quitting adjuvant chemotherapy, and death due to adjuvant chemotherapy. Transitions among the 5 states were assumed to be Markovian. Pw-w, Pw-mi, Pw-ma, Pmi-w, Pmi-mi, Pmi-ma, Pma-w, Pma-mi, Pma-ma, Pma-q, and Pma-d were applied to denote the probabilities of transition of the model in which suffix w represented the first state (well state), mi represented state of minor toxicity, ma represented state of major toxicity, q represented state of quitting the adjuvant chemotherapy, and d represented death state due to the adjuvant chemotherapy. Pw-mi represented the probability of the changing of patient in the well state in the current cycle to the minor toxicity state in the next cycle.
In this cost-effectiveness analysis, the authors compared XELOX and FOLFOX4 as adjuvant chemotherapy for patients with colon cancer based on data obtained from MOSAIC and No. 16968 trails from a Chinese cost-effectiveness perspective. The authors demonstrated that XELOX was expected to dominate FOLFOX4 regimens; in other words, XELOX was a more cost-effective treatment for adjuvant chemotherapy for patients with colon cancer in China.
P- Reviewer: Goetze TO S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9855] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 2. | Benson AB, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J, McAllister P, Van Cutsem E. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408-3419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1017] [Cited by in RCA: 1063] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 3. | Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1208] [Cited by in RCA: 1175] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 4. | Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet. 1995;345:939-944. [PubMed] |
| 5. | Wolmark N, Rockette H, Mamounas E, Jones J, Wieand S, Wickerham DL, Bear HD, Atkins JN, Dimitrov NV, Glass AG. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol. 1999;17:3553-3559. [PubMed] |
| 6. | André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2653] [Cited by in RCA: 2722] [Article Influence: 129.6] [Reference Citation Analysis (0)] |
| 7. | André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1502] [Cited by in RCA: 1638] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 8. | Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K, Schmoll HJ. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 571] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 9. | National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology-Colon cancer-v. 3. 2013; Available from: http://www.nccn.org/ professionals/physician_gls/f_guidelines.asp. |
| 10. | Shiroiwa T, Takeuchi T, Fukuda T, Shimozuma K, Ohashi Y. Cost-effectiveness of adjuvant FOLFOX therapy for stage III colon cancer in Japan based on the MOSAIC trial. Value Health. 2012;15:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Aballéa S, Chancellor JV, Raikou M, Drummond MF, Weinstein MC, Jourdan S, Bridgewater J. Cost-effectiveness analysis of oxaliplatin compared with 5-fluorouracil/leucovorin in adjuvant treatment of stage III colon cancer in the US. Cancer. 2007;109:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Attard CL, Maroun JA, Alloul K, Grima DT, Bernard LM. Cost-effectiveness of oxaliplatin in the adjuvant treatment of colon cancer in Canada. Curr Oncol. 2010;17:17-24. [PubMed] |
| 13. | Shiroiwa T, Fukuda T, Tsutani K. Cost-effectiveness analysis of XELOX for metastatic colorectal cancer based on the NO16966 and NO16967 trials. Br J Cancer. 2009;101:12-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Xie Q, Wen F, Wei YQ, Deng HX, Li Q. Cost analysis of adjuvant therapy with XELOX or FOLFOX4 for colon cancer. Colorectal Dis. 2013;15:958-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Ayvaci MU, Shi J, Alagoz O, Lubner SJ. Cost-effectiveness of adjuvant FOLFOX and 5FU/LV chemotherapy for patients with stage II colon cancer. Med Decis Making. 2013;33:521-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1882] [Cited by in RCA: 2041] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 17. | CTCAE 3. 0. 2006; Available from: http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. |
| 18. | Shiroiwa T, Sung YK, Fukuda T, Lang HC, Bae SC, Tsutani K. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19:422-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 513] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 19. | Ramsey SD, Andersen MR, Etzioni R, Moinpour C, Peacock S, Potosky A, Urban N. Quality of life in survivors of colorectal carcinoma. Cancer. 2000;88:1294-1303. [PubMed] |
| 20. | van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ. 1994;3:309-319. [PubMed] |
| 21. | Murray CJ, Evans DB, Acharya A, Baltussen RM. Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ. 2000;9:235-251. [PubMed] |
| 22. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11828] [Article Influence: 844.9] [Reference Citation Analysis (4)] |
| 23. | Jansman FG, Postma MJ, Brouwers JR. Cost considerations in the treatment of colorectal cancer. Pharmacoeconomics. 2007;25:537-562. [PubMed] |
| 24. | Schrag D. The price tag on progress--chemotherapy for colorectal cancer. N Engl J Med. 2004;351:317-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 314] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 25. | National Institute for Health and Clinical Excellence. Guide to the Methods of Technology Appraisal. London: National Institute for Health and Clinical Excellence 2008; . |
| 26. | Zhang F, Kong LL, Zhang YY, Li SC. Evaluation of impact on health-related quality of life and cost effectiveness of Traditional Chinese Medicine: a systematic review of randomized clinical trials. J Altern Complement Med. 2012;18:1108-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |