Published online Dec 21, 2014. doi: 10.3748/wjg.v20.i47.17737
Revised: September 26, 2014
Accepted: November 18, 2014
Published online: December 21, 2014
Processing time: 202 Days and 7.9 Hours
The incidence of type 2 diabetes (T2DM) is rapidly increasing worldwide. However, the pathogenesis of T2DM has not yet been well explained. Recent evidence suggests that the intestinal microbiota composition is associated with obesity and T2DM. In this review, we provide an overview about the mechanisms underlying the role of intestinal microbiota in the pathogenesis of T2DM. There is clear evidence that the intestinal microbiota influences the host through its effect on body weight, bile acid metabolism, proinflammatory activity and insulin resistance, and modulation of gut hormones. Modulating gut microbiota with the use of probiotics, prebiotics, antibiotics, and fecal microbiota transplantation may have benefits for improvement in glucose metabolism and insulin resistance in the host. Further studies are required to increase our understanding of the complex interplay between intestinal microbiota and the host with T2DM. Further studies may be able to boost the development of new effective therapeutic approaches for T2DM.
Core tip: Type 2 diabetes (T2DM) is believed to be caused by a series of multiple risk factors such as genetic liability, age, overweight or obesity, and an unhealthy lifestyle. Recently, accumulated evidence has suggested that the intestinal microbiota plays an important role in the pathogenesis of T2DM as a potential novel contributor. This review focuses on the underlying role of intestinal microbiota in the pathogenesis of T2DM and the therapeutic potential of modulating gut microbiota in T2DM.
- Citation: Han JL, Lin HL. Intestinal microbiota and type 2 diabetes: From mechanism insights to therapeutic perspective. World J Gastroenterol 2014; 20(47): 17737-17745
- URL: https://www.wjgnet.com/1007-9327/full/v20/i47/17737.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i47.17737
According to recent estimates by the International Diabetes Federation, there are 382 million people living with diabetes worldwide, and the number is expected to rise to 592 million by 2035[1]. Nearly 85%-95% of people with diabetes have type 2 diabetes (T2DM)[2]. T2DM is believed to be caused by a series of multiple risk factors such as genetic liability, age, overweight or obesity, and an unhealthy lifestyle. Recently, accumulated evidence has suggested that the intestinal microbiota plays an important role in the pathogenesis of T2DM as a potential novel contributor.
The adult human intestine is colonized by about 100 trillion bacteria, which is about 10 times the number of total cells in the human body[3]. Recent evidence suggests that the intestinal microbiota composition is associated with obesity and T2DM. Ley et al[4] analyzed 5088 bacterial 16S rRNA gene sequences from the gut microbiota of obese ob/ob mice and their lean control group. They found that ob/ob mice had a 50% decrease in the abundance of Bacteroidetes and a proportional increase in Firmicutes. They also observed similar differences in the gut microbiota of obese compared with lean humans[5]. Intestinal microbiota compositional changes have also been investigated in patients with T2DM. Researchers have found that the abundance of Firmicutes and Clostridia was significantly reduced, while the relative proportion of Bacteroidetes and Betaproteobacteria was increased in the diabetic group compared with the control group[6]. However, Zhang et al[7] found that the proportion of Firmicutes and Clostridia were higher in the group of patients with T2DM compared to the normal glucose group. Patients in the pre-diabetes and T2DM groups had a significantly increased level of Betaproteobacteria compared with the normal glucose group. Qin et al[8] have developed a protocol for a metagenome-wide association study based on deep shotgun sequencing of the gut microbial DNA extracted from fecal samples from Chinese T2DM patients and nondiabetic controls. They identified 47 metagenomic linkage groups in the T2DM-associated gene markers from the gut metagenome. Their results showed that patients with T2DM had a moderate degree of gut microbial dysbiosis, a reduction in the abundance of some butyrate-producing bacteria, and an increase in various opportunistic pathogens. Karlsson et al[9] observed significantly higher levels of four Lactobacillus species and significantly lower levels of five Clostridium species in the T2DM group. Importantly, these changes did not correlate with body mass index (BMI), waist circumference, or waist-to-hip ratio. Sato et al[10] showed that stool samples of diabetic patients had significantly reduced levels of the Clostridium coccoides group, Atopobium cluster, and Prevotella, and a significantly increased level of total Lactobacillus compared with control subjects. They also noted that the detection rate of live gut bacteria in the blood of diabetic patients was significantly higher than that in control subjects (28% vs 4%, P < 0.01). These studies that have aimed to evaluate the association between gut microbiota and diabetes have produced conflicting results. There may be many factors influencing the results, such as race, eating habits, geographical location, and research methods. This review focuses on the underlying mechanism of intestinal microbiota in the pathogenesis of T2DM, and the therapeutic potential of modulating the gut microbiota in T2DM.
Humans do not have the enzymes necessary for digestion of many types of plant polysaccharide, such as cellulose, xylans, resistant starch, and inulin[11]. However, these indigestible carbohydrates can be fermented by intestinal microbes to yield energy and to produce short-chain fatty acids (SCFAs). The role of the intestinal microbiota in the regulation of host body weight and energy homeostasis was revealed primarily in rodents. Bäckhed et al[12] and his colleagues found that conventionally raised mice had 42% more total body fat than germ-free mice (raised in the absence of any microorganisms), even if their daily caloric intake was 29% less than their germ-free counterparts. The germ-free mice transplanted with fecal microbiota from conventionally raised animals for 14 d had a 57% increase in their total body fat. In further investigation, the fecal gross energy content of lean conventionally raised C57BL/6J mice was significantly higher than in their obese counterparts (ob/ob mice)[13]. Germ-free mice transplanted with fecal microbiota from obese donors had a significantly greater increase in total body fat than those colonized with microbiota from lean donors. These results confirmed that the intestinal microbiota in ob/ob mice was more effective in harvesting energy from food than that of their lean littermates.
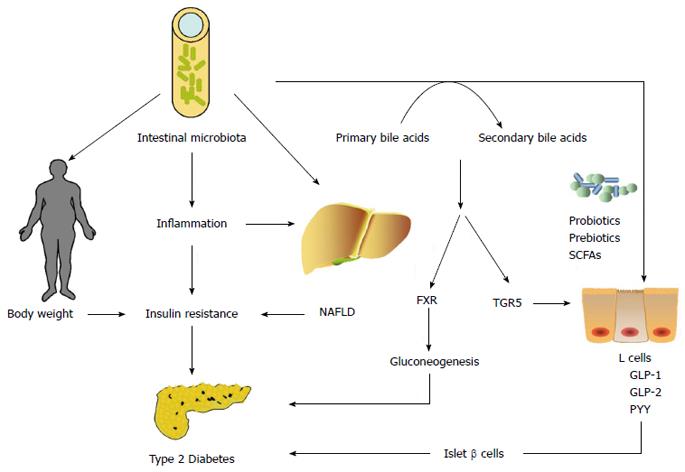
Cholic acid and chenodeoxycholic acid are the primary bile acids synthesized from cholesterol in the liver in humans. Once the primary bile acids, such as cholic acid and chenodeoxycholic acid, have reached the intestine, they may be transformed by intestinal microbiota into secondary bile acid species: deoxycholic and lithocholic acids[14]. The intestinal microorganisms strongly affect bile acid metabolism. The most typical secondary bile acid and the most abundant bile acid in biliary bile in humans is deoxycholic acid, which is converted from cholic acid via a 7α-dehydroxylation reaction catalyzed by some species of Clostridium in the large intestine[15]. Compared with germ-free mice, conventionally raised (CONV-R) mice have significantly lower levels of bile acid in the gallbladder and small intestine, but significantly higher levels of bile acid in the cecum, colon, feces, and serum. The total amount of bile acid was 71% lower in CONV-R than germ-free mice. Activation of the nuclear farnesoid X receptor (FXR) by the gut microbiota reduce the expression levels of most bile acid synthesis enzymes[16]. In turn, bile acids contribute to suppression of bacterial colonization and growth in the gut because of their strong antimicrobial activity. A previous study showed that the primary mechanism underlying the antimicrobial action of bile acids was membrane damage[17]. Only microbial populations able to tolerate physiological concentrations of bile acids can survive in the gut. Feeding with cholic acid induces phylum-level alterations in the composition of the gut microbiota in rats. Cholic acid feeding increases significantly the ratio of Firmicutes to Bacteroidetes, which is similar to the changes induced by high-fat feeding[18].
Over the past decade, a growing body of evidence has shown that bile acids play an important role in glucose metabolism as signaling molecules and cellular receptor ligands. Bile acids activate not only FXR but also the membrane-bound, G-protein-coupled receptor (GPCR) 1 (also known as TGR5)[19]. It has been demonstrated that bile acids inhibit the expression of gluconeogenic genes, such as those encoding phosphoenolpyruvate carboxykinase, fructose-1, 6-biphosphatase-1, and glucose-6-phosphatase in vitro via FXR[20]. Knocking out FXR in ob/ob mice prevented diet-induced obesity and improved murine hyperglycemia and glucose tolerance by increasing peripheral glucose clearance and adipose tissue insulin sensitivity[21]. Bariatric surgery, such as vertical sleeve gastrectomy (VSG), is effective for treatment of obesity and comorbid T2DM. FXR contributes to the maintenance of weight loss and improvement in glucose tolerance following VSG, which are associated with increased circulating bile acids and transition in gut microbiota composition[22]. Activation of TGR5 in enteroendocrine L cells induces glucagon-like peptide (GLP)-1 release, with an improvement in liver and pancreatic function and increased glucose tolerance in obese mice[23]. Activation of TGR5 in brown adipose tissue and muscle increases energy expenditure and alleviates diet-induced obesity[24].
Fasting serum taurine-conjugated bile acid concentrations are higher in T2DM compared with normoglycemic controls, and intermediate in impaired glucose tolerance. This pattern is not directly linked to obesity and glucose per se[25]. However, increases in taurine-conjugated bile acid in patients with T2DM may be related to lower rates of taurine deconjugation that is catalyzed by some bile salt hydrolases enriched in the human gut microbiota[26]. Bile-acid sequestrants have been used to sequester bile acids in the intestine to increase bile acid synthesis and consequently reduce serum low-density lipoprotein cholesterol. Bile-acid sequestrants have also been demonstrated to improve glucose control in patients with T2DM, which might be through multiple mechanisms such as altering the bile acid pool composition, improving hepatic glucose metabolism, increasing release of incretin hormones, and inducing changes in gut microbiota composition[27,28].
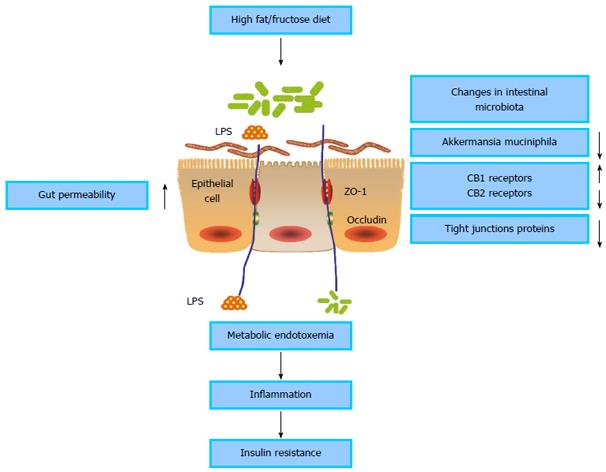
Obesity, insulin resistance and T2DM are closely associated with low-grade inflammation characterized by disordered cytokine production and activation of a network of inflammatory signal pathways[29,30]. The low-grade inflammation is induced by a change in gut microbiota that promotes metabolic endotoxemia and triggers the development of inflammation via a lipopolysaccharide (LPS)- and CD14/toll-like receptor (TLR) 4-dependent mechanism[31] (Figure 1). The intestinal microbiota is enriched with molecules such as LPS and peptidoglycan that may cause inflammation. Gut microbiota-derived LPS is involved in the onset and development of inflammation and metabolic diseases[32]. Elevated plasma LPS concentration in mice induced by high-fat feeding is defined as metabolic endotoxemia. Moreover, a high-fat diet in mice significantly alters intestinal microbiota composition. Metabolic endotoxemia is induced in mice through continuous subcutaneous infusion of LPS for 4 wk, and fasted glycemia and insulinemia, weight gain and expression of inflammatory cytokines are increased similarly to those in mice fed a high-fat diet. CD14 knockout mice resist most of the LPS and high-fat diet-induced characteristics of metabolic diseases. CD14 plays a key role in innate immunity[33]. The binding of LPS to the complex of mCD14 and TLR4 at the surface of the innate immune cells activates the cascade reaction of inflammation[34,35]. Many typical proinflammatory stimuli, including LPS, lipids, fatty acids, and chemokines activate c-Jun N-terminal kinase (JNK) and IκB kinase (IKK)-β pathways intracellularly. IKKβ activation stimulates the family of nuclear factor (NF)-κB transcription factors and increases the expression of numerous mediators of inflammation that can cause insulin resistance. JNK activation promotes the phosphorylation of insulin receptor substrate (IRS)-1 at serine sites, which inhibits normal signal transduction through the insulin receptor/IRS-1 axis, which also can result in insulin resistance[36]. Therefore, the metabolic endotoxemia induced by LPS derived from the gut microbiota is associated with inflammation and insulin resistance.
Increased endotoxemia is correlated with increased gut permeability. A high-fat diet in mice dramatically increases gut permeability and reduces expression of tight junction proteins such as zonula occludens (ZO)-1 and occludin in intestinal epithelial cells. Antibiotic treatment reduces metabolic endotoxemia in high-fat-fed mice, which is associated with decreased gut permeability, reduced inflammatory markers, and improved metabolic features of diabetes and obesity. Furthermore, the deficiency in CD14 in ob/ob CD14 knockout mice demonstrates the metabolic and inflammatory effects similar to those of antibiotics[37]. Intestinal permeability in human T2DM detected by the 51Cr-EDTA urinary recovery test was significantly increased compared with matched control subjects[38]. Modulating gut microbiota composition with prebiotics improves gut permeability, reduces metabolic endotoxemia, lowers inflammation, and alleviates glucose intolerance[39,40].
The endocannabinoid (eCB) system is now believed to be associated with inflammation and diabetes[41,42]. Intestinal microbiota modulate gut eCB expression, which controls gut permeability and plasma LPS levels through the CB1 receptor[43]. Changes in the gut microbiota due to prebiotic feeding reduce gut permeability in obese mice. Blocking the CB1 receptor in obese mice also improves gut barrier function by increased distribution and localization of tight junction proteins (ZO-1 and occludin). This demonstrates that the eCB system modulates gut permeability through the distribution and localization of tight junction proteins[44]. Bermudez-Silva et al[45] have shown that cannabinoid CB2 receptor activation improves glucose tolerance in rats and that CB1 receptor blockade mimics the effects of CB2 receptor agonists. The data suggest that the eCB system modulates glucose homeostasis through the interplay of CB1 and CB2 receptors. The changes in CB2 receptor expression are correlated positively with intestinal counts of Lactobacillus supplement and negatively with counts of Clostridium supplement[46]. Modulation of the intestinal microbiota with specific probiotics has been shown to upregulate CB2 receptor expression in rodents[47].
Akkermansia muciniphila (A. muciniphila) is a mucoprotein-degrading bacterium that colonizes the mucous layer[48], and its abundance is negatively correlated with body weight in humans[49,50]. The abundance of A. muciniphila decreases in obese and T2DM mice. Feeding with viable A. muciniphila ameliorates high-fat-diet-induced metabolic disorders, including adiposity, metabolic endotoxemia, low-grade inflammation, and insulin resistance. Feeding also promotes intestinal expression of eCBs that control inflammation, gut barrier, and gut hormone secretion. However, these effects are not observed in the same mouse model fed with heat-killed A. muciniphila[51].
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of liver disorders, including steatosis, nonalcoholic steatohepatitis (NASH), fibrosis and cirrhosis, which is often associated with obesity, insulin resistance, and diabetes mellitus. The prevalence of ultrasonographic NAFLD in patients with T2DM ranged from 54.11% to 78% in different studies[52]. A two-hit mechanism has been proposed for the pathogenesis of NAFLD. The first hit is the process of triglyceride accumulation in hepatocytes, while the second hit is responsible for hepatocyte injury, inflammation, and fibrosis through oxidative stress, lipid peroxidation, and proinflammatory cytokines[53,54]. Accumulated evidence has suggested that intestinal microbiota may be associated with NAFLD progression. Bäckhed et al[12] observed that colonization of germ-free mice with normal intestinal microbiota induced insulin resistance and stimulated hepatic lipogenesis. Gut microbiota compositional change can increase the amount of TLR ligands delivered to the liver. TLR ligands stimulate Kupffer cells and hepatic macrophages to produce proinflammatory cytokines that can result in insulin resistance and hepatocyte death[55]. In addition to TLRs, nucleotide-binding oligomerization domain-like receptors (NLRs) are inflammasome-dependent pathways of proinflammatory cytokine production[56,57]. Henao-Mejia et al[58] demonstrated that the NLRP6 and NLRP3 inflammasomes and the effector protein interleukin-18 negatively regulated NAFLD/NASH progression through modulation of the gut microbiota. A meta-analysis to evaluate the effects of probiotic therapy in NAFLD showed that probiotic therapies can improve liver function, lipid metabolism, and insulin resistance in NAFLD patients[59]. A recent randomized clinical trial demonstrated that 4-mo supplementation with probiotics significantly improved fatty liver in children with NAFLD[60].
GLP-1 is an incretin secreted from intestinal L cells. GLP-1 has numerous physiological effects, including stimulation of glucose-dependent insulin secretion, augmentation of β-cell mass, and inhibition of glucagon release, gastric emptying, and food intake[61]. Yadav et al[62] modulated the gut flora composition of mice with a probiotic, VSL#3. The altered gut microbiota stimulated production of SCFAs (butyrate) that promoted GLP-1 secretion from L cells to improve the metabolic state. SCFA activation of GPCR GPR41 and GPR43 promotes the secretion of GLP-1[63]. Prebiotics are non-digestible dietary ingredients that cause specific gut microbial composition changes or stimulate selectively the activity of some microbial species. Many investigations have demonstrated that prebiotics increase release of GLP-1 and improve the metabolic inflammation and insulin resistance induced by a high-fat diet[64-66].
GLP-2 is co-secreted with GLP-1 and is able to enhance intestinal epithelial proliferation and reduce gut permeability[67]. Changes in mouse gut microbiota with prebiotic ingestion promote a significant release of plasma GLP-2 levels and improve systemic and hepatic inflammation. GLP-2 receptor blockade impairs prebiotic-induced improvement in inflammatory tone[39]. Besides the roles of GLP-2 in maintaining gut barrier integrity, slowing gastric emptying and intestinal motility, improving nutrient absorption, and enhancing immune function, GLP-2 in central neurons enhances hepatic insulin sensitivity and plays a key role in the control of glucose homeostasis[68].
Peptide YY (PYY) is a gastrointestinal hormone secreted from intestinal L cells. PYY has several biological actions including vasoconstriction, inhibition of gastric acid secretion, reduction of pancreatic and intestinal secretion, regulation of appetite and inhibition of gastric motility[69,70]. Rats receiving a diet supplemented with oligofructose, oligofructose-enriched inulin or high-molecular-weight inulin demonstrated an increase in portal serum levels of GLP-1 and PYY. The same effects were also observed in diabetic rats[71]. Dietary-resistant starch is a fermentable fiber that liberates SCFAs through fermentation in the gut. Feeding rats with dietary-resistant starch increases GLP-1 and PYY secretion in the lower gut through SCFAs[72].
A modulating effect of the gut microbiota on T2DM was suggested by recent observations. Probiotics are nonpathogenic live microorganisms that may confer health benefits on the host. In an animal study, researchers observed that a fermented milk product containing probiotic bacteria significantly delayed the onset of glucose intolerance, hyperglycemia, and hyperinsulinemia in diabetic rats induced by high fructose concentration[73]. In elderly T2DM patients who consumed a daily dose of 200 mL of a symbiotic drink containing 108 CFU/mL Lactobacillus acidophilus, 108 CFU/mL Bifidobacterium bifidum and 2 g oligofructose over over 30 d, there was a significant increase in high-density lipoprotein cholesterol and a significant reduction in fasting glycemia[74]. In another investigation, patients with T2DM who consumed 300 g/d of probiotic yogurt containing L. acidophilus La5 and Bifidobacteriumlactis Bb12 for 6 wk had a significant reduction in fasting glycemia and hemoglobin A1c[75].
Antibiotic treatment is another method of gut microbiota modulation. Treatment with norfloxacin and ampicillin (1 g/L each) for 2 wk, suppressed the numbers of cecal bacteria in ob/ob mice. The treated animals displayed a significant improvement in fasting blood glucose and oral glucose tolerance. The enhanced insulin sensitivity was independent of food intake, weight loss, or adiposity. In this study, both plasma LPS levels and the expression of jejunal tumor necrosis factor-α level were significantly lower in the antibiotic-treated mice than in the control mice, suggesting that modulation of intestinal microbiota by antibiotics ameliorated the inflammatory status in ob/ob mice[76]. When diet-induced obese and insulin resistant mice were treated with the non-absorbable antibiotics polymyxin B and neomycin, they had a gradual reduction in glycemia, associated with a modified cecal microbiota profile[77]. Berberine, one of the main ingredients of a Chinese traditional herb used to treat bacterial diarrhea, improves glycemia. The antimicrobial activity of berberine and its modulation of the gut microbiota may play a role in the antidiabetic effect of this herb[78]. However, long-term use of antibiotics in humans is related to weight gain and obesity. Patients who receive 6 wk intravenous treatment with vancomycin plus gentamicin for infective endocarditis show significant weight gain[79]. Hernández et al[80] observed that intravenous β-lactam therapy for 14 d promoted glycosidase activity in the human gastrointestinal tract and was associated with BMI and glucose level. These data presented an interesting view of the potential effects of antibiotics on human metabolism. Further studies should be performed to investigate the effects of different antibiotics and administration routes on metabolism and T2DM.
Recently, a report about fecal microbiota transplantation has aroused strong interest. Fecal microbiota transplantation was testified to be a highly successful therapy for recurrent Clostridium difficile infection[81] This also raised interest in the therapeutic effect of fecal transplantation in metabolic syndrome and T2DM. Patients with metabolic syndrome who received small intestinal infusions of fecal microbiota from allogenic lean donors for 6 wk showed an improvement in peripheral and hepatic insulin sensitivity, along with an increase in butyrate-producing intestinal microbiota[82].
Intestinal microbiota may play an important role in the pathogenesis of T2DM by influencing body weight, bile-acid metabolism, proinflammatory activity and insulin resistance, and modulation of gut hormones (Figure 2). Modulating the gut microbiota through the use of probiotics, prebiotics, antibiotics, and fecal microbiota transplantation may have benefits in improving glucose metabolism and insulin resistance in the host. However, there are still many questions that need to be resolved. LPS inhibits the synthesis of insulin in isolated rat islets of Langerhans through binding of TLR4 and activation of the NF-κB pathway[83]. We do not know whether the changes in intestinal microbiota directly influence the β cell mass and function of islets in T2DM patients. Can we detect gut bacterial genes in liver or islets in T2DM patients? Does bariatric surgery for obese T2DM patients interact with their intestinal microbiota? Rifaximin, an oral locally acting antibiotic used to treat inflammatory bowel disease, can modulate gut microbiota. What effect of rifaximin can we observe if it is administered to T2DM animal models or patients? Future studies are required to increase our understanding of the complex interplay between intestinal microbiota and the host with T2DM, and to enable the development of new effective treatments for T2DM.
P- Reviewer: Bernardo WM, Ferrer M, Freedberg DE, Grundmann O, Keller JJ S- Editor: Ma YJ L- Editor: Cant MR E- Editor: Liu XM
| 1. | International Diabetes Federation. IDF Diabetes Atlas, Sixth Edition. 2013; Available from: http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf. [Cited in This Article: ] |
| 2. | Prevention of diabetes mellitus. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;844:1-100. [PubMed] [Cited in This Article: ] |
| 3. | Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4:430-435. [PubMed] [Cited in This Article: ] |
| 4. | Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070-11075. [PubMed] [Cited in This Article: ] |
| 5. | Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023. [PubMed] [Cited in This Article: ] |
| 6. | Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1783] [Cited by in F6Publishing: 1972] [Article Influence: 140.9] [Reference Citation Analysis (0)] |
| 7. | Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One. 2013;8:e71108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 481] [Cited by in F6Publishing: 576] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 8. | Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3971] [Cited by in F6Publishing: 4545] [Article Influence: 378.8] [Reference Citation Analysis (0)] |
| 9. | Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1766] [Cited by in F6Publishing: 1823] [Article Influence: 165.7] [Reference Citation Analysis (0)] |
| 10. | Sato J, Kanazawa A, Ikeda F, Yoshihara T, Goto H, Abe H, Komiya K, Kawaguchi M, Shimizu T, Ogihara T. Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care. 2014;37:2343-2350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 286] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 11. | Salyers AA, Gherardini F, O’Brien M. Utilization of xylan by two species of human colonic Bacteroides. Appl Environ Microbiol. 1981;41:1065-1068. [PubMed] [Cited in This Article: ] |
| 12. | Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718-15723. [PubMed] [Cited in This Article: ] |
| 13. | Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031. [PubMed] [Cited in This Article: ] |
| 14. | Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1046] [Cited by in F6Publishing: 1167] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 15. | Prabha V, Ohri M. Review: Bacterial transformations of bile acids. World J Microbiol Biotechnol. 2006;22:191-196. [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1367] [Cited by in F6Publishing: 1560] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 17. | Kurdi P, Kawanishi K, Mizutani K, Yokota A. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol. 2006;188:1979-1986. [PubMed] [Cited in This Article: ] |
| 18. | Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141:1773-1781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 596] [Cited by in F6Publishing: 645] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 19. | Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res. 2009;50:1509-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 527] [Cited by in F6Publishing: 501] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 20. | Yamagata K, Daitoku H, Shimamoto Y, Matsuzaki H, Hirota K, Ishida J, Fukamizu A. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J Biol Chem. 2004;279:23158-23165. [PubMed] [Cited in This Article: ] |
| 21. | Prawitt J, Abdelkarim M, Stroeve JH, Popescu I, Duez H, Velagapudi VR, Dumont J, Bouchaert E, van Dijk TH, Lucas A. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes. 2011;60:1861-1871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 22. | Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 694] [Cited by in F6Publishing: 726] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 23. | Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1223] [Cited by in F6Publishing: 1328] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 24. | Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484-489. [PubMed] [Cited in This Article: ] |
| 25. | Wewalka M, Patti ME, Barbato C, Houten SM, Goldfine AB. Fasting serum taurine-conjugated bile acids are elevated in type 2 diabetes and do not change with intensification of insulin. J Clin Endocrinol Metab. 2014;99:1442-1451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci USA. 2008;105:13580-13585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 591] [Cited by in F6Publishing: 709] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 27. | Sonne DP, Hansen M, Knop FK. Bile acid sequestrants in type 2 diabetes: potential effects on GLP1 secretion. Eur J Endocrinol. 2014;171:R47-R65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Hansen M, Sonne DP, Knop FK. Bile acid sequestrants: glucose-lowering mechanisms and efficacy in type 2 diabetes. Curr Diab Rep. 2014;14:482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860-867. [PubMed] [Cited in This Article: ] |
| 30. | Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1141] [Cited by in F6Publishing: 1290] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 31. | Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546-1558. [PubMed] [Cited in This Article: ] |
| 32. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [PubMed] [Cited in This Article: ] |
| 33. | Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res. 2005;11:225-229. [PubMed] [Cited in This Article: ] |
| 34. | Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431-1433. [PubMed] [Cited in This Article: ] |
| 35. | Sweet MJ, Hume DA. Endotoxin signal transduction in macrophages. J Leukoc Biol. 1996;60:8-26. [PubMed] [Cited in This Article: ] |
| 36. | Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793-1801. [PubMed] [Cited in This Article: ] |
| 37. | Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470-1481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3224] [Cited by in F6Publishing: 3365] [Article Influence: 210.3] [Reference Citation Analysis (0)] |
| 38. | Horton F, Wright J, Smith L, Hinton PJ, Robertson MD. Increased intestinal permeability to oral chromium (51 Cr) -EDTA in human Type 2 diabetes. Diabet Med. 2014;31:559-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 39. | Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091-1103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1735] [Cited by in F6Publishing: 1794] [Article Influence: 119.6] [Reference Citation Analysis (1)] |
| 40. | Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, Possemiers S, Van Holle A, François P, de Vos WM. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60:2775-2786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 755] [Cited by in F6Publishing: 781] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 41. | Scherer T, Buettner C. The dysregulation of the endocannabinoid system in diabesity-a tricky problem. J Mol Med (Berl). 2009;87:663-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Cani PD, Geurts L, Matamoros S, Plovier H, Duparc T. Glucose metabolism: focus on gut microbiota, the endocannabinoid system and beyond. Diabetes Metab. 2014;40:246-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 43. | Muccioli GG, Naslain D, Bäckhed F, Reigstad CS, Lambert DM, Delzenne NM, Cani PD. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 447] [Cited by in F6Publishing: 479] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 44. | Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3:279-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 560] [Cited by in F6Publishing: 579] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 45. | Bermudez-Silva FJ, Sanchez-Vera I, Suárez J, Serrano A, Fuentes E, Juan-Pico P, Nadal A, Rodríguez de Fonseca F. Role of cannabinoid CB2 receptors in glucose homeostasis in rats. Eur J Pharmacol. 2007;565:207-211. [PubMed] [Cited in This Article: ] |
| 46. | Aguilera M, Vergara P, Martínez V. Stress and antibiotics alter luminal and wall-adhered microbiota and enhance the local expression of visceral sensory-related systems in mice. Neurogastroenterol Motil. 2013;25:e515-e529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 47. | Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13:35-37. [PubMed] [Cited in This Article: ] |
| 48. | Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54:1469-1476. [PubMed] [Cited in This Article: ] |
| 49. | Santacruz A, Collado MC, García-Valdés L, Segura MT, Martín-Lagos JA, Anjos T, Martí-Romero M, Lopez RM, Florido J, Campoy C. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104:83-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 593] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 50. | Karlsson CL, Onnerfält J, Xu J, Molin G, Ahrné S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring). 2012;20:2257-2261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 383] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 51. | Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066-9071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2639] [Cited by in F6Publishing: 3094] [Article Influence: 281.3] [Reference Citation Analysis (0)] |
| 52. | Firneisz G. Non-alcoholic fatty liver disease and type 2 diabetes mellitus: the liver disease of our age? World J Gastroenterol. 2014;20:9072-9089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 65] [Reference Citation Analysis (0)] |
| 53. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [PubMed] [Cited in This Article: ] |
| 54. | Duvnjak M, Lerotić I, Barsić N, Tomasić V, Virović Jukić L, Velagić V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J Gastroenterol. 2007;13:4539-4550. [PubMed] [Cited in This Article: ] |
| 55. | Miura K, Ohnishi H. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:7381-7391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 252] [Cited by in F6Publishing: 245] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 56. | Gangarapu V, Yıldız K, Ince AT, Baysal B. Role of gut microbiota: obesity and NAFLD. Turk J Gastroenterol. 2014;25:133-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 57. | Aron-Wisnewsky J, Gaborit B, Dutour A, Clement K. Gut microbiota and non-alcoholic fatty liver disease: new insights. Clin Microbiol Infect. 2013;19:338-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 58. | Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1620] [Cited by in F6Publishing: 1782] [Article Influence: 148.5] [Reference Citation Analysis (0)] |
| 59. | Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911-6918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 232] [Cited by in F6Publishing: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 60. | Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39:1276-1285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 298] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 61. | Gareth EL, Patricia LB. Glucagon-Like Peptide 1 Secretion by the L-Cell: The View From Within. Diabetes. 2006;55:S70-S77. [DOI] [Cited in This Article: ] |
| 62. | Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288:25088-25097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 453] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 63. | Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1524] [Cited by in F6Publishing: 1509] [Article Influence: 125.8] [Reference Citation Analysis (0)] |
| 64. | Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like Peptide-1. Obes Res. 2005;13:1000-1007. [PubMed] [Cited in This Article: ] |
| 65. | Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes. 2006;55:1484-1490. [PubMed] [Cited in This Article: ] |
| 66. | Delzenne NM, Cani PD, Neyrinck AM. Modulation of glucagon-like peptide 1 and energy metabolism by inulin and oligofructose: experimental data. J Nutr. 2007;137:2547S-2551S. [PubMed] [Cited in This Article: ] |
| 67. | Dubé PE, Brubaker PL. Frontiers in glucagon-like peptide-2: multiple actions, multiple mediators. Am J Physiol Endocrinol Metab. 2007;293:E460-E465. [PubMed] [Cited in This Article: ] |
| 68. | Shi X, Zhou F, Li X, Chang B, Li D, Wang Y, Tong Q, Xu Y, Fukuda M, Zhao JJ. Central GLP-2 enhances hepatic insulin sensitivity via activating PI3K signaling in POMC neurons. Cell Metab. 2013;18:86-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 69. | Batterham RL, Bloom SR. The gut hormone peptide YY regulates appetite. Ann N Y Acad Sci. 2003;994:162-168. [PubMed] [Cited in This Article: ] |
| 70. | Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides. 2012;46:261-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 330] [Cited by in F6Publishing: 330] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 71. | Delzenne NM, Cani PD, Daubioul C, Neyrinck AM. Impact of inulin and oligofructose on gastrointestinal peptides. Br J Nutr. 2005;93 Suppl 1:S157-S161. [PubMed] [Cited in This Article: ] |
| 72. | Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, Danna SC, Tripathy S, Hegsted M, Keenan MJ. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295:E1160-E1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 308] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 73. | Yadav H, Jain S, Sinha PR. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition. 2007;23:62-68. [PubMed] [Cited in This Article: ] |
| 74. | Moroti C, Souza Magri LF, de Rezende Costa M, Cavallini DC, Sivieri K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012;11:29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 75. | Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28:539-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 389] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 76. | Membrez M, Blancher F, Jaquet M, Bibiloni R, Cani PD, Burcelin RG, Corthesy I, Macé K, Chou CJ. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 2008;22:2416-2426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 368] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 77. | Chou CJ, Membrez M, Blancher F. Gut decontamination with norfloxacin and ampicillin enhances insulin sensitivity in mice. Nestle Nutr Workshop Ser Pediatr Program. 2008;62:127-137; discussion 137-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 78. | Han J, Lin H, Huang W. Modulating gut microbiota as an anti-diabetic mechanism of berberine. Med Sci Monit. 2011;17:RA164-RA167. [PubMed] [Cited in This Article: ] |
| 79. | Thuny F, Richet H, Casalta JP, Angelakis E, Habib G, Raoult D. Vancomycin treatment of infective endocarditis is linked with recently acquired obesity. PLoS One. 2010;5:e9074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 80. | Hernández E, Bargiela R, Diez MS, Friedrichs A, Pérez-Cobas AE, Gosalbes MJ, Knecht H, Martínez-Martínez M, Seifert J, von Bergen M. Functional consequences of microbial shifts in the human gastrointestinal tract linked to antibiotic treatment and obesity. Gut Microbes. 2013;4:306-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 81. | van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2582] [Cited by in F6Publishing: 2563] [Article Influence: 233.0] [Reference Citation Analysis (0)] |
| 82. | Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913-916.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1881] [Cited by in F6Publishing: 1886] [Article Influence: 157.2] [Reference Citation Analysis (0)] |
| 83. | Amyot J, Semache M, Ferdaoussi M, Fontés G, Poitout V. Lipopolysaccharides impair insulin gene expression in isolated islets of Langerhans via Toll-Like Receptor-4 and NF-κB signalling. PLoS One. 2012;7:e36200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |