Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Nov 14, 2014; 20(42): 15931-15936
Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15931
Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15931
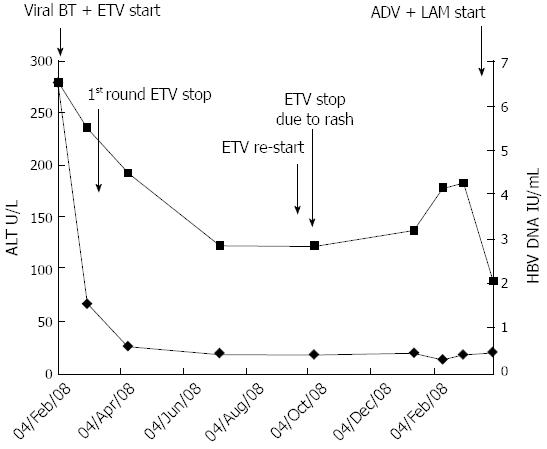
Figure 3 Alanine aminotransferase and hepatitis B virus DNA levels following the course of various antiviral treatments.
Entecavir (1 mg) treatment could not be continued past the 15th day due to an adverse drug reaction (skin rash). ALT: Alanine aminotransferas; HBV: Hepatitis B virus; ADV: Adverse drug reaction; BT: Breakthrough; ETV: Entecavir; LAM: Lamivudine.
- Citation: Kim JT, Jeong HW, Choi KH, Yoon TY, Sung N, Choi YK, Kim EH, Chae HB. Delayed hypersensitivity reaction resulting in maculopapular-type eruption due to entecavir in the treatment of chronic hepatitis B. World J Gastroenterol 2014; 20(42): 15931-15936
- URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15931.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15931