Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Nov 7, 2014; 20(41): 15018-15027
Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15018
Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15018
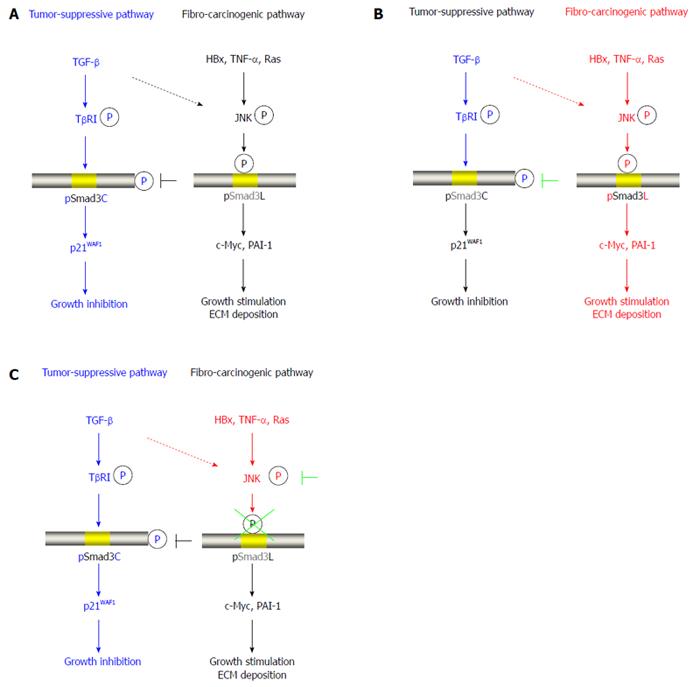
Figure 1 Reversibility of phospho-Smad3 signaling between tumor suppression and fibro-carcinogenesis.
A: Additional treatment of transforming growth factor-β (TGF-β) activates TGF-β receptor type I (TβRI), further leading to direct phosphorylation of Smad3C, which inhibits normally hepatocytic growth by upregulating p21WAF1 transcription; B: Mitogens drastically alter phospho-Smad3 signaling via the c-Jun N-terminal kinase (JNK) pathway, increasing nuclear fibro-carcinogenic pSmad3L activity while shutting down TGF-β-dependent cytostatic pSmad3C. Although the TGF-β signal weakly phosphorylates Smad3L in normal hepatocytes (dotted line), hepatitis viral components, including HBx, pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), and somatic mutations such as Ras, additively transmit a fibro-carcinogenic signal through the JNK-dependent pSmad3L pathway to participate in hepatocytic growth and ECM deposition, possibly by stimulating transcription of c-Myc and PAI-1 genes. Linker phosphorylation of Smad3 indirectly prevents COOH-tail phosphorylation, pSmad3C-mediated p21WAF1 transcription and cytostatic function; C: Either various JNK inhibitors or a Smad3 mutation causing lack of JNK phosphorylation sites in the linker region can eliminate fibro-carcinogenic pSmad3L signaling, restoring or maintaining the tumor-suppressive pSmad3C signaling characteristic of mature hepatocytes.
- Citation: Murata M, Yoshida K, Yamaguchi T, Matsuzaki K. Linker phosphorylation of Smad3 promotes fibro-carcinogenesis in chronic viral hepatitis of hepatocellular carcinoma. World J Gastroenterol 2014; 20(41): 15018-15027
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15018.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15018