Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Sep 28, 2014; 20(36): 12767-12780
Published online Sep 28, 2014. doi: 10.3748/wjg.v20.i36.12767
Published online Sep 28, 2014. doi: 10.3748/wjg.v20.i36.12767
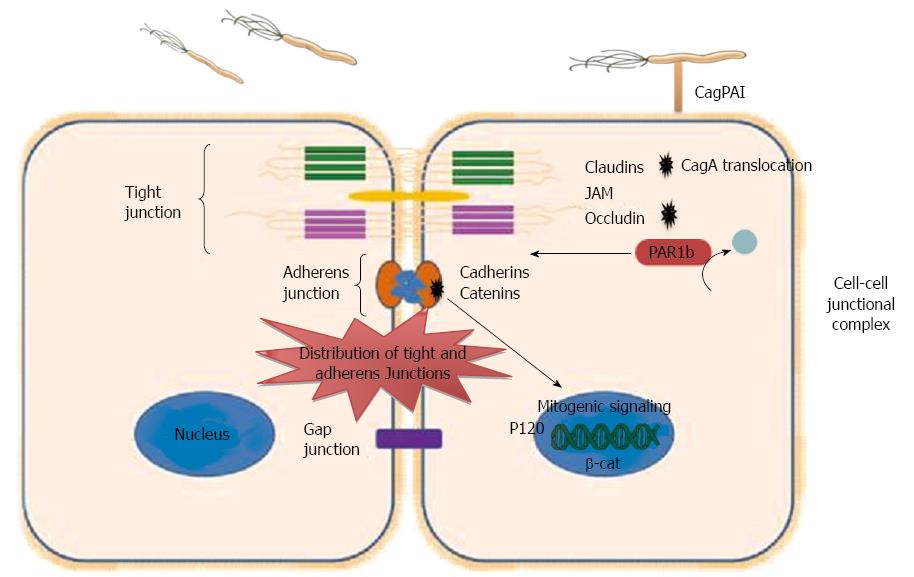
Figure 2 Dysregulation of epithelial junction by Helicobacter pylori.
Intercellular junctions include tight junctions, adherens junctions, and gap. Tight junction consists of important Integral membrane proteins, such as occludin, claudins, and junctional adhesion molecules (JAMs), while adheren junction consists of Catenins and Cadherins. Helicobacter pylori (H. pylori) interact with cell-cell junctions and disrupt the polarity of polarized epithelial cells. Translocated CagA binds to PAR1b and inhibits its activity and blocks its phosphorylation by atypical protein kinase C, eventually causing junctional and polarity defects. CagA interacts with E-cadherin leads to the release of E-cadherin from the E-cadherin/β-catenin adherent complex, results in buildup of β-catenin at the cytoplasm and nuclear translocation of β-catenin and p120, and activation of β-catenin-mediated signaling that induces transformation of gastric epithelial cells. CagPAI: Cag pathogenicity island.
-
Citation: Alzahrani S, Lina TT, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Effect of
Helicobacter pylori on gastric epithelial cells. World J Gastroenterol 2014; 20(36): 12767-12780 - URL: https://www.wjgnet.com/1007-9327/full/v20/i36/12767.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i36.12767