INTRODUCTION
Helicobacter pylori (H. pylori) is the main cause of chronic gastritis, gastroduodenal ulcers and gastric cancer[1]. Interestingly, H. pylori is responsible for about 90% of cases of peptic ulcer formation[2,3]. The World Health Organization classified H. pylori as a class I carcinogen because of the epidemiological link of H. pylori infection with a higher risk of development of gastric malignancy[4].
H. pylori infection is typically acquired during childhood and usually becomes a lifelong infection, if left untreated[1]. During H. pylori infection the host mounts an immune response, but this response fails to clear the infection and H. pylori successfully establishes a persistent infection leading to chronic inflammation. Multiple lines of evidence suggest that the immune response during H. pylori infection plays an important role in pathogenesis. H. pylori successfully establish a chronic infection by achieving a delicate balance between inducing immune responses and surviving in the inflammatory milieu by using an array of important virulence factors. In this review, we discuss innate and adaptive immune responses to H. pylori and the mechanisms by which H. pylori evades immune-mediated clearance.
INHIBITION OF INNATE IMMUNE RECOGNITION
Evasion of recognition by pattern recognition receptors
H. pylori evades the innate immune system by a variety of mechanisms. One of these mechanisms is avoidance of detection by pattern recognition receptors (PRR), which are proteins that recognize pathogen-associated molecular patterns (PAMP’s). PAMP’s include a large group of molecules that are part of microbes and can vary from microbial surface molecules to nucleic acids. When PRR’s recognize PAMP’s they induce several extracellular activation cascades such as the complement pathways and various intracellular signaling pathways, leading to inflammatory responses that are essential for clearance of pathogens[5].
H. pylori eludes identification by PRR’s by multiple methods, including: avoidance of recognition by Toll-like receptors (TLR’s) and inhibition of c type lectin (DC-SIGN) mediated signaling. To avoid recognition by TLR’s the bacterium modulates its surface molecules (including LPS and flagellin). Lipopolysaccharide (LPS) is a glycolipid found on the outer membrane of gram negative bacteria[6]. It has three distinct units: lipid A, which is responsible for the toxic effects; a core polysaccharide of five sugars linked through ketodeoxyoctulonate to lipid A; and the O-antigen, an outer polysaccharide consisting of up to 25 repeating units of three to five sugars[7]. H. pylori expresses O-antigens with great diversity; the bacterium has Lewis antigens, which are made of carbohydrates, that resemble human blood group antigens[8]. By exploiting this form of molecular mimicry, the bacterium is able to evade TLR’s because the normally detectable O-antigen is recognized as a “self” molecule by this type of PRR. In addition to having variable O-antigens, the bacterium also modifies the lipid A portion of the LPS molecule. Modification of this unit is achieved through several pathways, resulting in alteration of the net charge of the microbial surface. This leads to an inability of cationic antimicrobial peptides (CAMP’s) to bind to typically negatively charged structures like lipid A[6]. Lipid A, within LPS, is recognized by the human Toll-like receptor 4-myeloid differentiation factor 2 (hTLR4-MD2) complex. H. pylori expresses a modified Kdo (3-deoxy-D-manno-octulosonic acid)-lipid A structure tetra-acylated with a phosphoethanolamine added at the 1 position of the dissacharide[9], which might promote high resistance to CAMP and decreased activation of the hTLR4-MD2 complex[9]. Cullen et al[6] found that the 1 and 4’-phosphatases involved in lipid A synthesis in H. pylori act synergistically to produce a bacterial surface that is highly resistant to CAMP attack. In addition, dephosphorylation of H. pylori lipid A at the 1 and/or 4’ position results in LPS with attenuated hTLR4-MD2 activation. Moran[10] proposed that reduced immunogenicity of H. pylori LPS could be due to uncommon phosphorylation and acylation of H. pylori lipid A. H. pylori LPS binds poorly and at a slower rate to LPS-binding proteins, which are acute phase reactants that aid in LPS binding to CD14 and TLR4 on monocytes/macrophages. This reduced binding of LPS to its receptors results in decreased activation of monocyte-macrophages, preventing their contribution to innate immune response. Interestingly, H. pylori LPS has also been shown to possess anti-phagocytic properties in vitro[11].
Flagellin is the protein component of bacterial flagella needed for motility and colonization[12]. H. pylori rely on five or six polar flagella made of two separate subunits, FlaA and FlaB, to enable movement within the gastric mucus and to counteract peristalsis[13]. TLR5 is a PRR that recognizes flagellin. However, studies showed that H. pylori flagellin was not recognized by TLR5, and thus failed to induce nuclear factor (NF)-κB activation[14]. The study also reported that an 8 amino acid stretch in the N-terminal D1 domain of flagellin differed from that of flagellin from bacteria that activated TLR5. One study showed that flagellin, especially FlaA, is not “shed” by the bacteria and thus could not be detected by western blots in supernatants of infected gastric epithelial cells[13]. There were no evident traces of flagellin, which diminished the probability of it interacting with TLR5, allowing for evasion of this mechanism of bacterial recognition. Most flagellated bacteria are able to induce a proinflammatory state by promoting production of IL-8, but H. pylori flagellin seems unable to induce IL-8 production in GECs[13].
H. pylori LPS is important not only for the activation of TLR4 but also because the bacterium expresses Lewis (Le) blood group antigens in the O-antigen portion of the LPS molecule. As mentioned above, this polysaccharide area of the molecule is a clear method of evasion of the innate immune response because the Le group antigen system is biochemically related to carbohydrates present in ABO blood groups. The bacterium employs molecular mimicry to evade recognition by the innate immune system. The group of Le antigens is divided into type 1 (Lea and Leb) and type 2 antigens (Lex and Ley). Approximately, 80%-90% of H. pylori strains express Lex and/or Ley antigens, whereas gastric epithelial cells (GECs) also express Lex/y antigens[10,15]. H. pylori uses phase variation in the synthesis of LPS, including Le antigens. Phase variation in this context refers to a high frequency of LPS phenotype changes, like a reversible on-and-off switch, that results in loss/gain of certain LPS epitopes, as well as a heterogeneous population of LPS.
It has been suggested that although H. pylori LPS is not a strong activator of TLR4, it may be modulating the immune response via interactions with DC-specific ICAM3-grabbing non-integrin (DC-SIGN). DC-SIGN belongs to a subset of PRR’s termed C-type lectin receptors (CLR’s), which are involved in inducing specific genes within cells in response to pathogens as well as in modulating TLR signaling[16]. When CLR’s are expressed on dendritic cells (DCs) they detect carbohydrates like mannose, fucose, and glucan, which are common on bacterial surfaces. Ligand binding to these receptors initiates signaling pathways which induce phosphorylation of a subunit of the NF-κB complex and result in an increased rate of transcription of proinflammatory cytokine and chemokine mRNAs, such as IL-8[16]. DCs possessing these receptors are found on all mucosal surfaces as well as in lymphoid organs. Miszczyk et al[17] showed that H. pylori LPS was able to bind to recombinant human DC-SIGN in vitro and that this binding was abolished in the presence of monoclonal antibodies against the Le antigens and when fucose was added. By binding to this receptor it may be possible that the presence or absence of certain carbohydrates at the O-antigen end of the molecule could determine how DCs help T cells mature. Another independent study showed that Le+ variants from clinical isolates were able to bind to DC-SIGN and have effects on the polarization of the T cell response (Th1 vs Th2)[18]. It seems that H. pylori targets DC-SIGN to block a polarized Th1 response by phase-variable expression of Le antigens. In addition, this study provided evidence that H. pylori strains without Lex and Ley were able to evade recognition by DC-SIGN and possibly evade detection by any other mechanism[18].
Inhibition of phagocytic killing
H. pylori infection activates an inflammatory response in its host which leads to the recruitment of macrophages, neutrophils, and lymphocytes to the gastric tissue[19]. H. pylori can efficiently inhibit its own uptake by these professional phagocytes. This antiphagocytic phenotype depends on type IV secretion components encoded by the cytotoxin-associated gene pathogenicity island (cag PAI)[20,21]. Macrophages can engulf H. pylori, but the bacterium has developed mechanisms to avoid killing upon phagocytosis[22-24]. In a study where ingestion of H. pylori by human and murine macrophages was monitored using immunofluorescence and electron microscopy, H. pylori type I strains (cag PAI+ and vaculating toxin A+, VacA+) were shown to employ an unusual mechanism to avoid phagocytic killing. Once inside the macrophage, H. pylori actively delayed actin polymerization and phagosome formation. H. pylori-containing phagosomes then underwent extensive clustering and fusion resulting in the formation of “megasomes” containing multiple bacteria, which caused resistance to intracellular killing[22,25]. Studies also showed enhanced survival of H. pylori type I strains in macrophages compared to type II strains, which lack cag PAI and VacA. H. pylori type I strains were shown to reside in compartments with early endosome properties and did not fuse with lysosomes. The study also showed that retention of TACO, a tryptophan aspartate-containing coat protein on phagosomes, inhibited fusion of phagosomes and lysosomes in macrophages infected with H. pylori type I strains. It is worth noting that VacA alone plays a significant role in the interruption of the phagosome maturation[24]. By interfering with the phagosome function, VacA might prevent phagocytic killing of H. pylori. In fact, a study showed that, by interfering with endosomal traffic, VacA altered the presentation of antigens by B cells[26]. This mechanism would be expected to result in impaired adaptive responses as presentation of antigens to T cells is critical for the initiation of protective immune responses, as will be described in detail below. A related recent study provided evidence that the effects of VacA on endosomal traffic may prevent the development of a strong Th1 response. The study showed that H. pylori VacA could redirect the endocytic pathway of the probiotic bacterium Lactobacillus acidophilus, which induces a polarized Th1 response, and does this by blocking the induction of key innate cytokines such as IFN-β and IL-12[27]. Like other pathogenic bacteria, H. pylori also regulate host trafficking pathways by the selective modification of GTPases in macrophages during infection. H. pylori has been shown to disrupt the actin cytoskeleton by suppressing Rgs1/2, Fgd2, and Dock8 which are the key regulators of the Rho, Rac, and Cdc42 GTPases, respectively[27]. These are required for the organization and dynamics of actin cytoskeleton needed for proper cell function. This is another mechanism that disrupts phagocyte function and helps H. pylori survival in its host[27].
Inhibition of killing by reactive oxygen species and nitric oxide
A major proinflammatory factor produced by H. pylori is neutrophil-activating protein (NAP)[28]. H. pylori-NAP (HP-NAP) is a 150 kDa oligomeric protein, which increases adhesion of PMNs to endothelial cells, stimulates phagocyte chemotaxis, and activates NADPH oxidase to produce reactive oxygen species (ROS)[29,30]. However, H. pylori produces catalase and superoxide dismutase to detoxify ROS[31,32]. H. pylori can also down-regulate CXCR1 and CXCR2 expression in human neutrophils, which act as receptors for the neutrophil recruiting chemokine, IL8; thus, resulting in an inhibitory effect on neutrophil migration and reduced bacterial killing[33]. H. pylori also disrupt NADPH oxidase targeting, which was shown to result in the release of superoxide anions in the cytoplasmic membrane instead of the accumulation inside H. pylori phagosomes[34].
One antimicrobial host defense mechanism is the generation of NO through the enzyme inducible NO synthase (iNOS). H. pylori activates the inducible iNOS in macrophages[35]. A mechanism employed by H. pylori to activate iNOS involves urease, an important virulence factor of H. pylori. Despite the presence of iNOS H. pylori infection persists, which suggests that the iNOS production may be at suboptimal level. H. pylori arginase was shown to be an important factor that affords protection of the bacteria against NO mediated killing since macrophages infected with H. pylori produce significantly less NO than arginase isogenic mutants[36]. A recent study showed that induction of macrophage arginase II (Arg2) restricts iNOS protein expression, elicits apoptosis of macrophages as well as proinflammatory cytokine production, and limits bacterial killing[37], suggesting another mechanism this bacteria uses to escape macrophage-mediated killing. Interestingly, the authors of that study used a chronic infection mouse model to show that Arg2-/- mice infected with H. pylori had reduced bacterial colonization and increased gastritis compared with similarly infected wild type mice. Arg2-/- mice infected with H. pylori had more iNOS+ macrophages in the gastric mucosa expressing higher levels of iNOS and more robust cytokine responses, which led to the suggestion that H. pylori induction of Arg2 is part of the bacterial armamentarium to escape host innate immunity together with other mechanisms that target adaptive immunity[37].
MODULATION OF APC FUNCTIONS IN ADAPTIVE IMMUNITY
H. pylori have evolved an array of mechanisms to actively dodge adaptive immunity by interfering with antigen presentation and modulation of T cell responses. Antigen presenting cells (APC), represented by macrophages, DCs and B cells, internalize antigen by phagocytosis or endocytosis, process the antigens and present them to CD4+ T cells via class II MHC molecules. This leads to the initiation of antigen specific T cell response. The gastric mucosa of H. pylori infected people has an increase in activated macrophages and DCs. Activated macrophages produce IL-6, IL-1β, IL-12 and tumor necrosis factor (TNF)-α which cause inflammation and help initiate Th1 type responses. In spite of the presence of these effector cells, H. pylori successfully establish a persistent infection, suggesting that these effector cells are unable to clear the pathogen. H. pylori has also been shown to cause the polarization of APCs. For instance, during atrophic gastritis macrophages are polarized to M1 subtype[38]. H. pylori can even control the functions of these APCs differently. A study showed that H. pylori mediated activation of DCs and M1 macrophage leads to induction of T cell proliferation and decreased phagocytosis. On the other hand, upon H. pylori infection the M2 macrophages produced less pro-inflammatory cytokines and increased anti-inflammatory cytokines compared to M1 macrophages[39]. As alluded to earlier, several studies show that H. pylori uses mechanisms to avoid killing by APC and those will be discussed in detail below.
Apoptosis of macrophages
H. pylori causes apoptosis of macrophages using several mechanisms. Inside a macrophage, H. pylori activate the ERK1/2 pathway leading to formation of the activation protein (AP-1) complex. AP-1 complex induced c-Myc gene expression and nuclear translocation leading to increased ornithine decarboxylase (ODC) expression and apoptosis of macrophages[40-42]. Another recent study showed an important role for an unknown gene, HP986, which is associated with peptic ulcer and gastric carcinoma in apoptosis of macrophages through a Fas mediated pathway[40]. H. pylori VacA protein also causes apoptosis of monocytes. The underlying mechanism of this process involves the amino-terminal 476 residue fragment (p52) of VacA, which activates the NFκB pathway and induces proinflammatory cytokine production, e.g., TNF-α, IL-1β, and induction of NO, ROS and subsequently causes apoptosis of monocytes[43].
Inhibition of DC maturation and function
DCs are important APCs in initiating T cell responses, particularly to mucosal pathogens. H. pylori control maturation of DCs and, consequently, limit their ability to present antigens. Transcription factor E2F1, is an important regulator of DC maturation. Using LPS as a stimulator, it was shown that E2F1 expression is downregulated during DC maturation. However, H. pylori VacA was shown to inhibit DC maturation via restoration of E2F1 since transfection of murine DCs with E2F1 siRNA showed recovery of the inhibited maturation of DCs caused by H. pylori VacA[44]. VacA caused reduced expression of surface costimulatory molecules, e.g., CD40, CD80, CD86, MHC class II molecule and decreased secretion of IL-1β, IL-12p70 and TNF-α by DCs[44]. Reduced expression of costimulatory molecules could, in turn, dampen effector T cell activation or promote tolerance. In addition to VacA, H. pylori CagA also plays a key role in regulating DCs and in inhibiting CD4+ T cells’ differentiation towards Th1 type cells. Once inside the APC, CagA protein was shown to be phosphorylated leading to the activation of SHP-2. Activated SHP-2 was shown to suppress the enzymatic activation of TBK-1, IRF-3 phosphorylation and nuclear translocation, which caused reduced interferon production by DC[45]. Long term infection with H. pylori cagA+ strains caused increased expression of the T cell co-inhibitory molecule B7-H1 (also known as PDL-1) as well as increased IL-10 and IL-23 production by DC. The simultaneous inhibition of DC maturation and IL-12 secretion led to suboptimal Th1 development and activation[46]. H. pylori were shown to multiply in DC and impair their function by inhibiting the production of the proinflammatory cytokine IL-12 and increasing IL-10 production[47]. A separate study showed that H. pylori-mediated inhibition of DC maturation is independent of the presence of cag PAI, but direct contact with the bacteria was required for this inhibitory mechanism[48]. When expression of different co-stimulatory molecules was evaluated after treating DCs with TLR ligands and subsequent infection with H. pylori it was found that H. pylori inhibit TLR ligand induced DC maturation by inhibiting CD80, CD86, CD40 expression, IL-12, IL-6 secretion and increased production of the anti-inflammatory cytokine IL-10[48]. These studies showed that inhibition of DCs is another mechanism used by H. pylori to deter its clearance by the host immune system.
Inhibition of antigen presentation
The proliferation of human CD4+ T cells is triggered by recognition of antigenic epitopes bound to MHC class II molecules exposed on the surface of APCs. Antigen presentation by APCs plays an essential role in the initiation of adaptive immune responses. As another approach to inhibit APC function, H. pylori use several mechanisms to interrupt antigen presentation, some of which were mentioned above. H. pylori inhibits antigen processing by APC by interfering with late endocytic membrane trafficking. H. pylori VacA interferes with proteolytic processing of antigens and the generation of T cell epitopes loaded on newly synthesized MHC class II molecules (the Ii-dependent pathway of antigen presentation), but it does not affect generation and presentation of epitopes by mature class II molecules that recycle from the cell surface (Ii-independent pathway)[26]. Also, possibly linked directly to this, H. pylori can also cause impaired antigen presentation by DC by inhibiting export of MHC-class II molecules to the cell surface[47]. This observation is directly related to the inability of these DCs exposed to H. pylori VacA to degrade Ii (aka, CD74), which requires the action of cathepsins activated by acidic pH[49-51].
Apoptosis of gastric epithelial cells
GEC may act as non-professional APCs, as they express all the elements associated with conventional APCs, including class II MHC, CD74, cathepsins and costimulatory molecules[52,53]. Their importance in H. pylori infection is obvious as they are the first cell types that come in direct contact with the bacteria and are strategically situated to interact with H. pylori and its antigens as well as with lamina propria lymphocytes. In fact, GEC separate the lamina propria immune cells from direct contact with H. pylori in the lumen. One of the multiple ways H. pylori has been shown to induce apoptosis of GEC is by upregulation of Fas receptor leading to increased interaction with Fas ligand and increased apoptosis[54]. This interaction has also been shown to induce production of ROS[55]. Another mechanism that our group showed previously is via the engagement of MHC class II molecules on GEC by H. pylori, which use urease on its surface as an adhesin and bind to and crosslink MHC class II molecules to induce apoptosis in GEC[56]. In yet another mechanism of induction of GEC apoptosis, VacA was shown to induce apoptosis via disruption of mitochondrial membranes[57,58].
Using GEC as orchestrators of T cell responses
Activation of T cells requires two signals triggered by (1) recognition by T cell receptor (TcR) of peptides/MHC complexes; and (2) a costimulation by special receptor molecules on APCs. Recognition of antigen by T cells in the absence of the second signal renders T cells unresponsive or anergic. The B7 family of costimulatory/coinhibitory receptors provides this second signal to initiate responses and some members of this family of receptors serve to regulate or attenuate responses. In addition to their role as on/off switches for T cell activity, recent studies from multiple groups also suggest their role in influencing T cell differentiation and phenotype[59-61]. Our group showed that H. pylori can subvert GECs and use them as mediators to inhibit T cell proliferation and cause Treg cell induction from naïve T cells by inducing increased expression of the T cell co-inhibitory molecule B7-H1 on GEC[62,63]. Interaction of B7-H1 with programmed death-1 (PD-1) receptor is also known to cause downregulation of T cell activation and promote the induction of T regulatory cells (Treg) as we have previously shown[62,63]. Because of their suppressive effect on T effector cells, Treg cells may assist in the chronicity of infection (Figure 1). H. pylori-mediated B7-H1 upregulation also contributes to apoptosis of effector T cells by engaging PD-1 on their surface[64]. Recently, our group uncovered another mechanism by which H. pylori uses its cagPAI encoded type 4 secretion system, T4SS, translocated effector protein CagA to downregulate B7-H2 (ICOS-L), which is the only positive T cell costimulatory molecule known among the newer members of this family of receptors. Downregulation of B7-H2 caused decreased Th17 cell response, which correlated with increased bacterial load in the stomach of H. pylori-infected mice[65]. As Th17 cells play a very important role in immune protection against extracellular bacteria, H. pylori hinders Th17-mediated clearance by preventing B7-H2 expression on the surface of GECs to establish chronic infection (Figure 2).
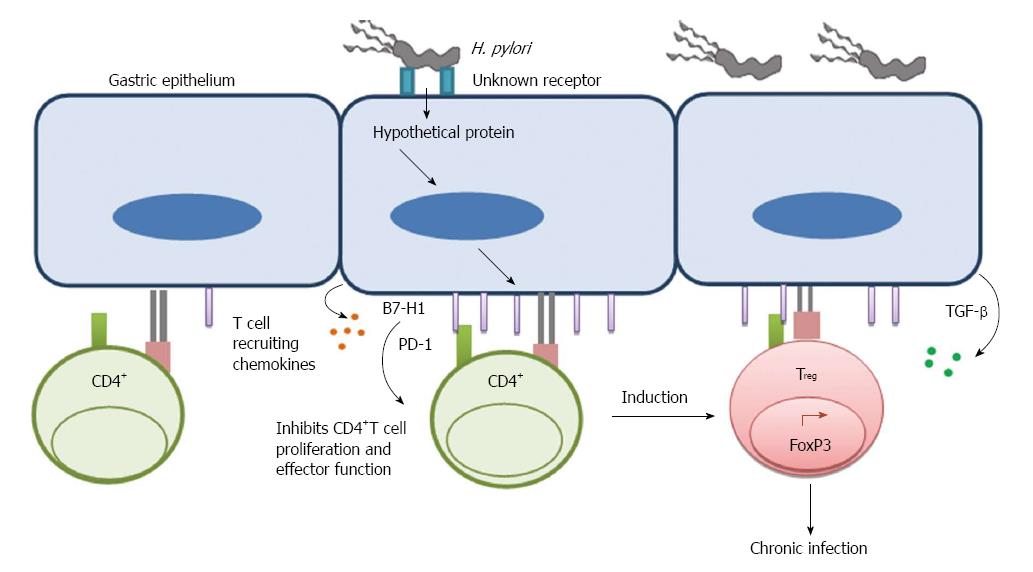
Figure 1 Helicobacter pylori uses gastric epithelial cells as a mediator to inhibit T cell function.
Upon binding to an unknown receptor on GEC Helicobacter pylori translocate a hypothetical protein, which causes induction of B7-H1 on GEC. Induction of T cell co-inhibitory molecule B7-H1 further inhibits CD4+ T cell proliferation and effector function. It also facilitates induction of Treg cells from naïve CD4+ T cells. This mechanism helps to establish a chronic infection.
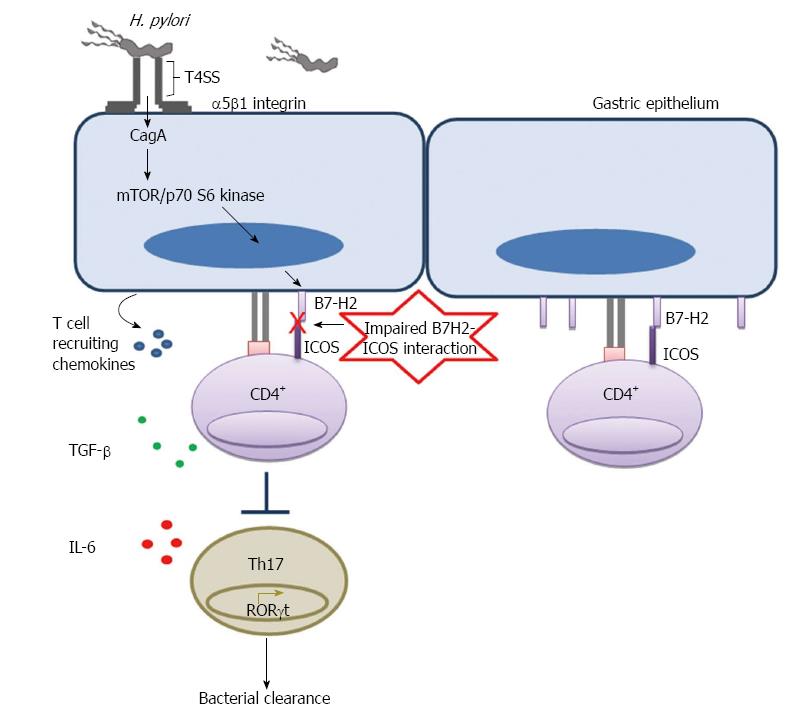
Figure 2 Helicobacter pylori mediated downregulation of B7-H2 on gastric epithelial cell inhibits Th17 cell development and facilitates bacterial persistence.
Helicobacter pylori T4SS interacts with host receptor integrin α5β1 and translocate effector protein CagA. CagA activates mTOR/p70 S6 kinase pathway and downregulates T cell co-stimulatory molecule B7-H2 expression on GEC. Decreased B7-H2/ICOS signaling further inhibits Th17 cell development from naïve CD4+ T cells and Th17 cell mediated bacterial clearance.
INHIBITION OF EFFECTIVE T CELL RESPONSE
T helper CD4+ cells (Th) are major effector cells in the immune response to H. pylori. The response was initially characterized as a Th1 polarized response[66,67] but more recently other CD4+ T cell subsets have been found in H. pylori-infected patients, and those include Treg and Th17 cells[68-71]. The neutrophil-activating protein of H. pylori (HP-NAP) was shown to increase IL-12 and IL-23 production by neutrophils and monocytes, which promote Th1 responses. Addition of HP-NAP to antigen-induced T cell lines caused a shift from a predominant Th2 to a Th1 phenotype of specific T cells. HP-NAP also elicited an antigen-specific Th1-polarized T cell response in the gastric mucosa of H. pylori-infected patients[72]. Increased production of gamma interferon (IFN-γ) by Th1 cells was shown to cause chronic gastric inflammation[66,73]. On the other hand increased Treg cells produced during H. pylori infection suppress mucosal effector T cell responses, which contribute to bacterial persistence, and are also a probable cause of gastric tumor progression[68]. Th17 cells, which produce IL-17A, appear to be crucial in the clearance of extracellular bacteria such as H. pylori[74]. IL-17 also acts on GEC to release IL-8, a chemokine that recruits neutrophils, and thus promote gastric inflammation. On the other hand, this IL-17-initiated recruitment of neutrophils is critical for the clearance of the bacteria[75]. A hallmark of H. pylori infection is that effector T cell responses are generally impaired during H. pylori infection, and T cells from H. pylori-infected individuals are hyporesponsive[76]. As this is an important issue in vaccine design efforts, there has been significant effort to address mechanisms that impair T cell responsiveness. H. pylori virulence factors that have been reported to play a role in interfering with T cell responses are VacA, γ-glutamyltranspeptidase (GGT), and arginase[77-82]. Recently our group showed H. pylori CagA also plays important role in modulating Th17 cell response indirectly by modulating expression of B7-H2 on GEC[65].
Inhibition of T cell proliferation and signaling
As described earlier, the vacuolating cytotoxin, VacA, induces cellular vacuolation in epithelial cells. H. pylori disrupt tight junctions between GECs and VacA secreted by H. pylori can reach the lamina propria. Once in the lamina propria, VacA can interact directly with T cells. H. pylori exploit the recycling of the heterodimeric transmembrane receptor lymphocyte function-associated antigen 1 (LFA-1) by cells for VacA uptake[73]. VacA enters activated primary human T lymphocytes by binding to the β2 (CD18) integrin subunit of LFA-1[83]. Once VacA is inside the cytoplasm of T cells it inhibits their proliferation and activation using several mechanisms. One approach is by interrupting IL-2 signaling, which is required for lymphocyte activation and proliferation. H. pylori VacA induces cell cycle arrest[84]. VacA also blocks IL-2 at the transcription level by inhibiting nuclear translocation of nuclear factor of activated T cells (NFAT), an essential transcription factor required for IL-2 promoter activation[84]. Further study into the mechanism of action of VacA on T cell impairment showed that VacA requires its intact N-terminal hydrophobic domain for membrane channel formation and inhibition of T cell proliferation[80]. Furthermore, VacA may reduce the mitochondrial membrane potential of CD4+ T cells to inhibit their proliferation[81]. In addition to preventing calcium influx from the extracellular milieu by formation of anion-specific channels and inhibiting NFAT translocation, VacA also uses a channel independent mechanism to activate intracellular signaling via mitogen-activated protein kinases MKK3/6 and p38 as well as the Rac-specific nucleotide exchange factor, Vav, which results in actin rearrangement and defect in T cell activation[77]. VacA mediated apoptosis in T cells is another possible mechanism of immune evasion. There are two pathways of apoptosis initiation. One pathway depends on death receptor and is called the extrinsic pathway; the second pathway depends on mitochondrial activation and is known as the intrinsic pathway. H. pylori mediated apoptosis of T cells is independent of death receptor. A mitochondrial pathway was shown to play a critical role in H. pylori induced apoptosis since higher expression of antiapoptotic protein Bcl-2 in T cells showed reduced apoptosis by H. pylori. Bcl-2 inhibits apoptosis by stabilizing the mitochondrial membrane[78].
GGT is another secreted protein of H. pylori which mediates the extracellular cleavage of glutathione, leading to ROS production and induction of a cell cycle arrest in lymphocytes. A study showed that H. pylori uses GGT to inhibit T cell proliferation since site directed mutagenesis of GGT in different H. pylori strains and inhibition of GGT by acivicin abrogated the inhibitory effect, while recombinant expression of GGT showed inhibition of T cell proliferation. GGT was found to inhibit T cell proliferation by inducing G1 cell cycle arrest through disruption of Ras signaling pathway[79].
Though most studies have shown involvement of VacA and GGT in T cell inhibition, other reports also showed involvement of cag PAI in T cell apoptosis. H. pylori cag PAI causes apoptosis in T cells in a Fas-dependent manner concurrently with induction of Fas ligand (FasL) in T cells leading to apoptosis[82]. Another virulence factor that impairs T cell function during H. pylori infection is arginase, which is important for urea production. Arginase hydrolyzed L-arginine to urea and ornithine. L-arginine is also required for T cell activation and function. Co-culturing of H. pylori wild type and arginase mutant bacteria with T cells revealed that arginase caused a significant decrease in T cell proliferation and reduced expression of the chief signal transduction protein CD3ζ-chain of the TCR by decreasing L-arginine availability[85]. Decreased expression of CD3ζ-chain partially explains T cell anergy status in the host, which is a hallmark of H. pylori infection.
H. pylori mediated skewing of T cell response towards Treg cells
CD4+CD25high Treg cells can inhibit infection-induced immunopathology, but may also allow for an increase in the bacterial load and facilitate chronicity of the infection by suppressing protective immune responses[86]. Treg cells are found in increased amounts in the gastric tissue of H. pylori infected patients compared to healthy controls[86,87]. Several studies have shown the immunosuppressive roles of Treg cells during H. pylori infection[63,86]. Induction of Treg cells appears to depend on the age of the host when they get the infection, since H. pylori infected children have increased levels of FoxP3-expressing Treg cells and reduced gastric pathology compared to adults[88]. A study in mice showed that mice that were infected during the neonatal period were protected from gastritis and gastric cancer precursor lesions in spite of having increased bacterial loads while adult mice infected with H. pylori developed those lesions. Neonatally infected mice developed tolerance, were unable to induce T cell responses and were protected from T cell-mediated immunopathology[89]. In contrast to Treg cells, Th17 cells play a crucial role in H. pylori clearance as suggested by vaccine studies[90,91]. However, H. pylori may utilize several mechanisms to induce Treg responses while keeping a suboptimal level of Th17 cells in the host, which helps to establish a chronic infection. There is an increased recruitment of DCs in the gastric lamina propria of H. pylori-infected mice[92]. A study by Kao et al[93] showed that DCs stimulated with H. pylori inhibits the Th17 response and skew the response toward Treg cells. This mechanism depends on development of Treg cells with the required cytokines, TGF-β and IL-10, and this mechanism was independent of H. pylori virulence factors VacA and CagA. This study further showed that Treg depletion enhanced the H. pylori specific Th17 response, and correlated with decreased bacterial colonization in mice[93]. In another mechanism of T cell suppression, H. pylori interfere with DC maturation process and convert immature DCs to tolerogenic DCs. Increased numbers of these semi-mature DCs were found in the chronically infected gastric mucosa of human H. pylori carriers. H. pylori-induced tolerogenic DCs were incapable of activating effector functions in naive T cells; however, these cells became very efficient in inducing Treg and this process depended on DC derived IL-18 production[48].
Bone marrow-derived mesenchymal stem cells (BM-MSCs) also play an important role in the H. pylori-induced immunosuppressive response. Transplantation of BM-MSCs into the stomach of mice with H. pylori infection fostered significant stimulation of systemic and local IL-10-secreting T cells, which may inhibit other T cells. There was also an increased percentage of CD4+IL-10+ cells and CD4+CD25+FoxP3+ cells in splenic mononuclear cells. BM-MSC-transplanted mice showed elevated Treg/Th17 ratios[94]. These studies showed that H. pylori uses several mechanisms to skew the T cell response towards Treg cells, which helps H. pylori to successfully establish a chronic infection.
EVASION OF HUMORAL RESPONSE
The majority of people infected with H. pylori develop a specific antibody response. This response is not normally enough to clear infection. Some studies suggest that infected children produce less antibodies, which may be concurrent with more Treg cells and less activated CD4+ cells to act as helper cells in the induction of B cell responses[95]. Although most or all infected individuals are thought to mount an antibody response to H. pylori, differences in this response have been noted between those who develop gastritis or duodenal ulcers compared to those who develop gastric cancer[96]. By examining patient serum antibody levels, infected individuals who developed gastritis or duodenal ulcers were shown to have a greater IgG response than those who developed gastric cancers. In turn, gastric cancer patients mounted a more vigorous IgA response than those with gastritis and duodenal ulcers. In another study of serum antibody responses to H. pylori in Japan, the authors suggested that a weak antibody response was linked to a high risk of developing gastric cancer by infected individuals[97]. Another study suggested that development of antibodies specific to virulence factors of H. pylori may be linked to gastric cancer[98]. In this study, gastric cancer patients were more likely to develop antibodies to CagA and heat shock protein B, while no significant differences were found in the levels of VacA specific antibodies between individuals with gastric cancer in comparison to other disease manifestations. These studies suggest that differences in humoral responses to infection may be linked to disease in infected individuals, but the mechanisms behind these differing responses remain elusive.
Although most people respond to H. pylori with a high serum antibody titer, this response is not efficient in reducing bacterial burden as evidenced by studies in mice that lack B cells and in various vaccine studies. In a study of mice lacking B cells, mice were protected against H. pylori challenge suggesting that the humoral response is dispensable in protection against H. pylori. To further support this study, another B cell knockout study showed that with an H. pylori urease vaccine, mice deficient in B cells had equal protection as wild type mice and stomach CD4+ T cells were equal in both mouse strains[99]. This study further indicated a correlation between the amount of T cells in the gastric mucosa and the level of protection, again suggesting that the humoral response is less crucial in protection against H. pylori. In addition to the viewpoint that protection against H. pylori challenge is independent of the B cell response, there is also compelling evidence that antibodies elicited against H. pylori may be harmful to the host. One group has shown in mice that specific antibody responses to H. pylori may actually aid in bacterial colonization and impair other immune responses against H. pylori[100]. This study showed that T cells, not B cells, were responsible for gastritis induced by infection and suggested the possible role for antibodies in inhibiting host resistance to infection in showing improved elimination of bacteria in the absence of antibodies in B cell deficient mice. B cell deficient mice were able to clear bacteria at 12-16 wk post infection, whereas wild type mice still had a robust infection coupled with gastritis at this time point. Another compelling study showed that H. pylori evade antibody mediated recognition because of a lack of surface binding of host elicited antibodies[101]. This study consisted of incubating bacteria with sera from patients who had detectable antibody responses to H. pylori. There was very little binding of antibodies to the surface of the bacteria, thus indicating another way the host immune response may be evaded.
Another intriguing aspect of the humoral response to H. pylori are reports of autoantibodies that are induced during infection. These antibodies were against self-epitopes and potentially caused damage in the host. For instance, one group showed that H. pylori induced antibodies against parietal cells in the stomach, which persisted after bacterial eradication and were linked to intestinal metaplasia[102]. In support of these results, another study examined autoantibodies in infected patient sera, revealing a prevalence of autoantibodies during gastritis associated with gland destruction and stomach atrophy[103]. There has also been indication of disease-specific autoantibodies induced by H. pylori infection. A study in duodenal ulcers showed that autoantibodies impair gastric secretory functions[104]. Decreased acid secretion, but increased gastrin were seen along with increased gastritis. This was shown in 20% of duodenal ulcer patients coupled with a more severe disease manifestation. Likewise, detrimental effects of autoantibodies have been seen in gastric cancer as well. In a small panel of gastric cancer patients, spleen cells were isolated, immortalized with human hybridoma technologies, which allowed for characterization of 11 H. pylori induced autoantibodies that reacted with gastric cancer cell specific proteins[105]. Several of these antibodies stimulated gastric cancer cells to proliferate, interestingly enough, in contrast to normal epithelial cells. These studies represent an intriguing aspect to H. pylori immune evasion in humans that may still require further investigation to clarify the mechanisms involved.
GENOMIC DIVERSITY IN IMMUNE EVASION
H. pylori is one of the most genetically diverse bacterial species. Initial insights into this diversity were apparent when the first strains of H. pylori were first sequenced. When 26695 and J99 H. pylori strains were compared at the genome level, it was observed that 6% of the genome represented strain specific genes, which are mostly located in a region now referred to as the plasticity zone[106]. Since then, multiple other strains have supported the observation that such diversity occurs at the size of the genome, gene arrangement and alleles. The extensive genetic diversity of H. pylori is the result of high mutation rates and high recombination frequency[107,108]. An important virulence factor of H. pylori is encoded in a 37-kB segment of DNA referred to as the cag PAI. Since it was discovered, cag PAI is perhaps the most studied region of the H. pylori genome. An array of H. pylori isolates have been noted to differ in the rate with which they have the cag PAI in their genome[109], which was recently supported by a study that included 877 isolated from diverse populations and which highlighted the variability in the carriage of cag PAI by H. pylori strains. This cag PAI mostly encode an array of structural constituents of a bacterial T4SS in addition to a 128 kDa effector protein, CagA. When H. pylori adheres to GECs, CagA is translocated via the T4SS into the host cell cytoplasm where it becomes phosphorylated by host cell kinases and interacts with various signaling proteins[110,111]. As a result of the multiple interactions of CagA with host cell signaling proteins, multiple processes are affected leading to cell transformation. This effector protein also has a significant level of diversity, particularly in the C-terminal EPIYA repeat motifs where CagA is phosphorylated once it is inside the host cell. These EPIYA motifs differ between Asian and Western isolates. An interesting study of H. pylori isolates from experimentally infected mice and non-human primates showed that they have rearrangements in CagY of the T4SS[112], which in turn result in gain or loss of function in the H. pylori T4SS. These observations may be reflective of the overall variability in H. pylori strains, which in turn contribute to immune escape and the establishment of chronic infection.
IMMUNE SYSTEM BASED THERAPY
To get an effective immune response against H. pylori, T cells must be activated into an effector state. Co-stimulatory and inhibitory molecules regulates T-cell activation, and works as “immune checkpoints”. H. pylori have been shown to upregulate B7-H1 expression and downregulate B7-H2 expression on GEC and, thus, not only decrease the effector T cell response but also alter T cell sub-population balances by increasing Treg and decreasing Th17 cell numbers[63-65]. This novel information may permit control H. pylori infection by targeting the inhibitory receptor/ligand axis. The anti-PD-1 monoclonal antibody nivolumab, (also known as MDX-1106 or BMS-936558) and lambrolizumab have already been used in a phase I trial and have shown promising results in patients with melanoma and other cancers[113-115], and conceivably could have an effect in the outcome of H. pylori infection and/or immunization. Since we have shown previously that H. pylori uses mTOR/p70 S6 kinase pathway to downregulate B7-H2 expression[65], another approach to control H. pylori could involve the use of rapamycin, which inhibits this pathway. Analogues of this drug have been used already and shown promising results in renal cell carcinoma and breast cancer treatments[116]. The immune modulatory properties of this bacterium could be exploited therapeutically to control other diseases. For example, recombinant HP-NAP has been shown to be beneficial in the treatment of allergic diseases and immunotherapy of cancer due to its ability to induce Th1 responses. It was shown to inhibit the growth of bladder cancer[117]. HP-NAP has also been used as an immune modulating agent to suppress Th2 responses in allergic asthma and Trichinella spiralis infection[118,119].
CONCLUSION
H. pylori has been co-existing with human host for at least 30000 years[120]. During this long time of co-existence the bacteria has undergone evolutionary adaptation and established a comfortable niche in the human host. Unlike most other pathogenic bacteria which are cleared by the host adaptive immune response, H. pylori successfully establishes a persistent infection in its host in spite of the presence of vigorous innate and adaptive immune response. H. pylori evolved an array of mechanisms to evade both innate and adaptive immune responses. Host mediated immune response not only fails to clear the bacteria but also helps the bacteria for colonization by proving increased availability of adhesion places such as MHC II and CD74, both of these components are induced by IFN-γ and IL-8 during H. pylori infection[121,122]. H. pylori virulence factors VacA, HP-NAP, Cag T4SS have been shown to cause damage in the gastric epithelium which results in peptic ulcer or even gastric cancer, if left untreated. Bacterial virulence factors together with host factors determine the severity of disease. Though multiple studies have examined how the bacteria interact with its host there is still a lack of clear knowledge about how it avoids host mediated immune responses. Furthermore little is currently known about the role of T cell subsets in controlling H. pylori infection and associated immunopathogenesis, particularly in humans. A better understanding of the mechanisms it uses to evade or subvert host immune response is crucial to design a therapeutic or a successful vaccine to eliminate this highly prevalent and deadly pathogen.
P- Reviewer: Lin CJ, Linden SK, McGee DJ, Sasidharan S S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN