Published online Sep 21, 2014. doi: 10.3748/wjg.v20.i35.12407
Revised: March 19, 2014
Accepted: May 19, 2014
Published online: September 21, 2014
Processing time: 302 Days and 2.2 Hours
The potential clinical impact of enhancing antitumor immunity is increasingly recognized in oncology therapeutics for solid tumors. Colorectal cancer is one of the most studied neoplasms for the tumor-host immunity relationship. Although immune cell populations involved in such a relationship and their prognostic role in colorectal cancer development have clearly been identified, still no approved therapies based on host immunity intensification have so far been introduced in clinical practice. Moreover, a recognized risk in enhancing immune reaction for colitis-associated colorectal cancer development has limited the emphasis of this approach. The aim of the present review is to discuss immune components involved in the host immune reaction against colorectal cancer and analyze the fine balance between pro-tumoral and anti-tumoral effect of immunity in this model of disease.
Core tip: Immune reactions accompany all stages of colorectal carcinogenesis and cancer progression. Recent evidence has shown that innate immunity pathways play a fundamental role in maintaining colorectal epithelial homeostasis and confer antitumor protection. However an excessive and unresolved innate immune reaction is the base of chronic colitis which is a well-known risk factor for colorectal cancer. Once the tumor has developed a number of immune cells may either favorably take under control its growth (CD45RO+CD8+ T cells) or favor its progression and metastatic spread (T regulatory cells). A fine regulation of all antitumor immune components is therefore necessary to design a proper immune-based therapeutic approach in colorectal cancer care and prevention.
- Citation: Formica V, Cereda V, Nardecchia A, Tesauro M, Roselli M. Immune reaction and colorectal cancer: Friends or foes? World J Gastroenterol 2014; 20(35): 12407-12419
- URL: https://www.wjgnet.com/1007-9327/full/v20/i35/12407.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i35.12407
Colorectal cancer is the third leading cause of cancer death worldwide and it is estimated that 190000 new cases of and 35000 deaths from colorectal cancer will be recorded in 2014 in the United States[1]. It is now accepted that the original carcinogenetic event hits the stem cells lying at the base of the intestinal crypts[2], and in many cases a developing process from epithelial hyperplasia, through dysplasia and adenomatous polyposis, to malignant transformation occurs, with accumulating gene mutations underlying this phenomenon[3-5].
There is an interesting debate among several scientists and clinicians on the dual role of immune/inflammatory response in colon carcinogenesis[6]. A considerable amount of preclinical and clinical findings have showed that mucosal immune/inflammatory cells can either promote or inhibit colorectal cancer (CRC) cell growth.
According to the association with inflammatory processes, two types of CRC are recognized: Sporadic CRC (SCRC) that is thought to arise from intrinsic genetic instability with inflammation being subsequent to cancer onset, and inflammation-induced CRC (colitis-associated CRC, or CAC) that is initiated by chronic inflammatory bowel disease (IBD). Two major forms of IBD occur in humans, ulcerative colitis (UC) and Chron’s disease (CD), and both of them may associate with CRC development, with CD/CRC correlation being moderate, whereas UC patients have a well-defined elevated risk of cancer diagnosis[7,8]. The extension and duration of the chronic inflammation correlate with the entity of cancer risk, however, it is not fully understood why CRC occurs only in a minority of IBD patients.
Like most solid tumors, both SCRC and CAC exhibit immune/inflammatory infiltrates, referred to as ‘tumor-elicited inflammation/immunity’, and a differential effect on patient outcome has been observed for distinct immune ‘players’. As an example, CD4(+) T-helper 1 (T(H)1) cells and CD8(+) cytotoxic T cells seem to constitute a positive prognostic sign in CRC, whilst it has been shown that myeloid cells, T regulatory cells (Tregs) and T-helper interleukin (IL)-17-producing (T(H)17) cells can promote tumorigenesis and associate with a drastic decrease in disease-free survival in stage I/II CRC[9].
Finally many epithelial cancers develop proximally to microbial communities, which are physically separated from immune cells by an epithelial barrier. Baseline immune response to these commensals and perhaps the epithelial barrier deterioration induced by the tumor tissue with invasion of microbial products may substantially contribute to the extent and nature of the inflammatory/immune response seen in CRC[10].
It is conceivable that the type, degree and timing of inflammatory/immune infiltrates and related cytokines can have a pivotal role in the initiation and progression of colon carcinogenesis, response to standard antitumor therapy and patient clinical outcome.
In the present review, we describe recent findings on the link between inflammation/immunity and CRC and summarize the role of different tumor-infiltrating innate and adaptive immune cells in promoting, sustaining or restraining CRC growth.
A great amount of research has dealt with the possibility that intestinal infection by “carcinogenic” bacteria might be linked to colorectal cancer development, with the assumption that pro-tumoral effect of infective processes is associated with a persistent and unresolved inflammatory reaction triggered by certain microorganisms.
In presence of a colorectal cancer, it has been shown a growth advantage to a particular subset of opportunistic bacteria colonizing the intestine. In particular, the opportunistic pathogens Streptococcus spp have been put forward as co-causative tumor factor.
In the 1950s, reports were published indicating a possible association between streptococcal infection and carcinomas of the gastrointestinal tract[11]. Boleij et al[12] have recently reviewed 31 studies documenting overall a 65% probability of colorectal neoplasia in patients with Streptococcus Bovis infection. Not all streptococcus bacteria seem to have the same carcinogenic potential and only a subspecie of Streptococcus Bovis, the Streptoccucus gallolyticus gallolyticus, has been found to be strongly related to colorectal cancer[13].
The suggested pathogenetic model relies on the preferential colonization by S Gallotycus of preneoplastic adenomatous lesions as a result of favourable metabolic and nutritional changes occurring in the dysplastic epithelium microenvironment (in particular high concentrations of lactate and carbohydrates)[14]. Once S Gallolyticus infection of adenomatous sites is established, the bacterium induces an inflammatory reaction consisting of hyperexpression of (COX-2) pathway[15,16].
COX-2 determines sustained epithelial cell proliferation and inhibition of apoptosis and triggers neoangiogenesis[17]. High COX-2 levels have been detected in approximately 85% of colon carcinomas[18], and consistent evidence suggests a protective role for anti-COX drugs (i.e., non-steroidal anti-inflammatory drugs, such as aspirin) against colorectal cancer development and progression[19].
The use of microbiological tests for streptococcal infection in human samples (e.g., fecal or peripheral blood serological tests) has raised interest as a potential tool of early CRC detection. However, early attempt to diagnose colorectal neoplasia on the basis of Streptococcal colonization have proven of limited value. In particular, it is estimated that only half of colorectal neoplasia are colonized by S gallolyticus, moreover current serological assays exploit immune recognition of antigens expressed on S gallollyticus pilus, which are characterized by a wide sequence heterogeneity[20]. Results of serological testing for S gallolyticus with the multi-antigen approach produced sensitivity results between 16% and 43%[21].
The association between bacterial infection and CRC development was also studied in the context of chronic inflammation in patients with IBDs. Several commensals, such as Fusobacterium varium, Bacteroides vulgatus, Escherichia coli, Helicobacter Hepaticus and Clostridium Clostridioforme, are commonly isolated from the mucosa of IBD. It has been demonstrated that these bacteria can adhere to colonic epithelial cells and invade their cytoplasms, resulting in the release of tumor necrosis factor (TNF)-α, IL-8 and IL-6. All such cytokines may predispose to IBDs and CRC[22].
In particular, recent findings in mouse model of CAC have indicated that H. hepaticus and E.Coli promote chronic colitis and tumorigenesis with E. Coli inducing also the expansion of pathogenic viruses, fungi and parasites with genotoxic capabilities[23,24].
Overall these results indicate that colonisation of certain microorganisms in the background of predisposing factors contribute to CRC carcinogenesis. Their main effect seems to rely on fuelling protracted inflammatory reaction which is a well-known cause of cancer development, rather than a direct mutagenic attack of the host.
Innate immunity is the first-line defence against microbial attack on intestinal surface. Microbes are initially recognized by means of surface and intracellular receptors that link to conserved molecular patterns of microbial origin, the so called pattern recognition receptors (PRRs). Several types of PRRs have so far been identified in humans, but two classes of PRRs seem to play a major role in intestinal innate immunity: the cytoplasmic NOD (nucleotide-binding and oligomerization domain) and NOD-like receptors (NLRs) and the membrane-bound toll-like receptors (TLRs)[25,26].
PRRs function on both epithelial and stromal myeloid cells, and excessive and uncontrolled PRRs activation by commensal and pathogen bacteria has long been considered the base of permanent inflammation in IBDs and CAC[27]. CAC approximately accounts for 2%[28] of all diagnosed CRCs, whereas sporadic cancers (SCRC) cover about 95% of cases. Even though SCRCs are thought to progress through a carcinogenetic process different from that of CAC, that is distinctive multiple step genetic loss, involving initial mutation of APC and activation of beta-catenin and subsequent mutations of K-Ras, PIK3CA and TP53[29], some of SCRCs may hold an ‘inflammatory gene signature’ reminiscent of CAC genetic landscape.
Three remarkable researches have explored the role of PRRs in CAC development using a murine model of chemical induced colitis with dextran sulfate sodium, DSS, which recapitulates human IBDs, and of CAC induced with DSS and the addition of azoxymethane (AOM). These researches have called into question the univocal protumoral role of innate immunity as innate immunity pathways are active both in inflammatory cells of the lamina propria (macrophages, dendritic cells and neutrophils) and in epithelial cells and in the epithelial tissue they seem to favourably contribute to cell homeostasis and protect against colitis and CAC.
These studies were focused on the transmembrane receptors TLR-4 (recognizing lipopolysaccharide, LPS, of gram negative bacteria) and TLR-2 (recognizing lipoteichoic acid, LTA, of gram positive bacteria) and their related intracellular signal transducer Myeloid Differentiation 88 (MyD88), on the NOD1 and NOD2 receptors and their mediator Receptor-interacting protein 2 (RIP2), and on the NLR-P3 and its final molecular effectors caspase 1 and 12.
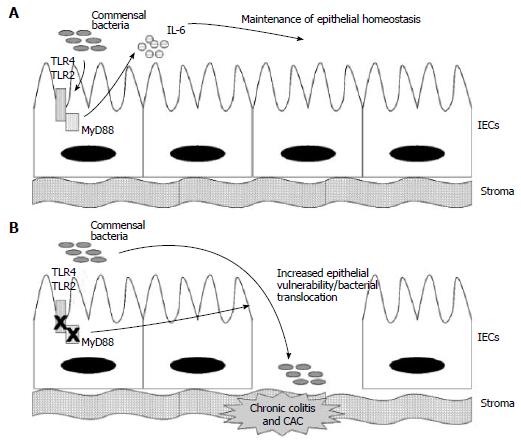
TLR-2/TLR-4/MYD88 axis: Rakoff-Nahoum et al[30] explored in 2004 the susceptibility to DSS-induced colitis of mice knockout for TLR4, TLR2 and MyD88, and for RIP2. They found that TLRs on Intestinal Epithelial Cells (IECs) are normally activated by commensal microorganisms and this symbiotic recognition is required for epithelial physiology. The initial hypothesis was that deficiency in TLRs pathway would determine attenuated inflammatory response and hence reduced colitis.
They unexpectedly observed in MyD88-/- mice severe colitis and increased mortality upon DSS administration, as compared to wild type (WT) mice, suggesting a protective role of MyD88-dependent axes. MyD88 is an essential mediator for many molecular cascades including those activated by TLRs, and receptors for IL-1 and IL-18. Authors decided to further explore the role of TLR2 and TLR4.
TLR2-/- or TLR4-/- partly reproduced MyD88-/- susceptibility to DSS colitis, with less severe clinical effects, thus inducing authors to conclude that MyD88 protective action was the result of influence on multiple molecular pathways including TLR4 and TLR2. They also investigated MyD88-independent inflammatory axes, in particular those dependent on the receptor-interacting protein (RIP)2, a mediator of cytoplasmic PRRs (the NOD1 and NOD2 receptors) which ultimately determines activation of the NF-κB transcriptional factor and MAP kinases, but found no increased susceptibility to DSS in RIP2-/- mice.
DSS induced limited damage in colon of WT mice that started from day 5 and resolved by day 12 with complete epithelial regeneration in 100% of cases. In the MyD88-/- mice, epithelial ulcers and erosions occurred as early as day 3, were more severe and persisted after day 12 with lethal effect, suggesting a role of MyD88 in regulating both epithelial resistance to injury initiation and post-injury epithelial repair. An uncontrolled bacterial overgrowth as the cause of severe DSS-colitis in MyD88 -/- mice was excluded as treatment with broad-spectrum antibiotics did not change the experimental results and, before DSS administration, there was no difference in faecal bacterial load between WT and MyD88 -/- mice. In an assay of intraperitoneal injection of Bromo deoxyuridine, more proliferating intestinal epithelial cells (IECs) were found in MyD88 -/- mice at baseline, but in the meantime, after DSS administration and injury, a defect in IEC proliferation and crypt repopulation was observed as compared to WT, indicating that a baseline hyperproliferative state may partly explain increased susceptibility to injury, but a reduced reparative capacity may account for the prolonged and lethal effect of DSS in these mice. MyD88 intervenes in the functioning of multiple molecular pathways and authors did not evaluate if the dual and opposite effect of MyD88 in cell cycle control derives from two distinct MyD88-dependent molecular signals.
Authors also explored MyD88-induced cytokines potentially responsible for epithelial resistance and regeneration, and in particular focused on IL-6, which had been found to contribute to gastrointestinal wound healing[31]. TLR/MyD88 axis regulates gene expression (including IL-6) via NF-κB transcriptional factor[32] and IL-6 was upregulated in WT mice (IL-6 flare) after DSS injury. Conversely, IL-6 upregulation was not seen in MyD88 deficient mice after DSS. Moreover, since antibiotic treatment abrogated baseline IL-6 production in WT mice, they argued that commensal flora determines physiological activation of MyD88 signal with induced IL-6 secretion, indispensable for epithelial regeneration after DSS injury.
IL-6 flare after DSS administration in WT mice, in fact, was abrogated by intense treatment with four antibiotics which sterilized from all commensal microflora, making these mice similar to MyD88-/- in that they displayed severe colitis and died after 12 d (Figure 1). However, Rakoff-Nahoum et al[30] did not investigate whether exogenous IL-6 administration was able to restore epithelial competence against DSS insult in this model. Moreover, it was not evaluated the cell type source of MyD88-induced IL-6. Stromal macrophages would be the most probable IL-6 suppliers, however also epithelial cells may express TLRs, MyD88 and IL-6 as suggested by a baseline IL-6 production in WT mice with intact epithelium.
Finally, the crucial interaction between MyD88 and TLR4 and TLR2 receptors was investigated in the Rakoff-Nahoum study. Experiments with TLR4-/- and TLR2-/- mice and with the specific agonists LPS for TLR4 and LTA for TLR2 were performed. They found that resistance to DSS-colitis was restored in mice completely-depleted by commensals if either LPS or LTA was co-administered. Specificity of these findings was confirmed by the fact that the rescue administration of LPS was ineffective in TLR4-/- and effective in TLR2-/-. This final finding suggests that one of the two axes (TLR4 or TLR2) is sufficient to maintain homeostasis, which is slightly in contrast with their initial observation that knockout mice for either TLR4 or TLR2 still suffer from severe DSS-induced colitis. Rakoff-Nahoum et al[30], moreover, did not evaluate if MyD88-/- model was also associated with increased tumorigenesis upon administration of DSS+AOM, however they affirmed that any component contributing to persistent inflammation would also potentially associate with high risk of cancer development. Rakoff-Nahoum’s results were reproduced in 2005 in a separate study by Araki et al[33].
NLRS/caspase 1/caspase 12/IL-18 axis: Dupaul-Chicoine et al[34] evaluated the role of caspase 1 and 12 on the risk of colitis and CAC. The molecular cascade terminating with caspase-1 inflammasome is a well-established pathway activated during colorectal inflammation both in mesenchymal and epithelial cells. Caspase-1 is the downstream target of a number of intracellular receptors sensing a potential harm,and in particular for NLRs, including those containing the pyrin domain (NLRPs), those containing caspase recruitment domain - 4 (NLRC4) and those inhibiting apoptosis. Caspase-1 ultimately determines the proteolytic cleavage of pro-IL1beta and pro-IL-18 in their respective active forms and induces an ‘inflammatory cell death’ named pyroptosis[35]. Caspase-1 is counterbalanced by caspase-12 which possesses inhibitory function on inflammasome activity.
At the end of this inflammatory cascade, IL-18 seems to play the more ‘articulated’ and complex role, with apparently contradictory evidence. Increased circulating and mucosal levels of IL-18 are associated with severe IBD[36]. Moreover in a study by Sivakumar et al[37] using the mouse model of DSS-induced colitis, a selective IL-18 inhibitor (the IL-18 binding protein IL-18bp.Fc) was able to reduce IBD manifestations (such as weight loss) and down-regulate many cytokine transcripts known to drive inflammation, including IL-1alpha, IL-1beta, TNF-alpha and IFN-gamma. In contrast to these observations, in humans genetic variants associated with reduced functionality of IL-18 axis are risk factors for IBD[38].
It is hypothesized that IL-18 plays a dual role: it maintains intestinal epithelial homeostasis by inducing epithelial cell regeneration and wound healing upon commensal microbial attacks under physiological conditions; while in pathological conditions, when the epithelial barrier is breached and chemical or microbiological colitis is initiated, it sustains the excessive inflammatory reaction within the stroma with increased risk for CAC[39].
Dupaul-Chicoine et al[34] analysed three genotypes of mouse (WT, caspase 1 -/- and caspase 12 -/-) and assessed their ability to repair colon tissue damage induced by DSS administration. As caspase 1 drives inflammasome formation and robust inflammatory responses, authors would have expected an attenuated colitis in Casp1-/- mice after DSS insult. Surprisingly, they found that caspase 1 has a crucial role in epithelial proliferation and regeneration after injury and, while on day 5 of DSS administration mice of all genotypes displayed epithelial erosion, by day 8, only WT mice had epithelial recovery and restitution, conversely casp1-/- displayed persistent signs of colitis, developed severe diarrhoea and rectal bleeding and all died by day 9. Interestingly, as well as wt mice, also Casp 12-/- recovered by day 9. However casp12-/- mice showed exaggerated tissue repair with hyperplastic crypts at the end of the experiment, suggesting a possible role for caspase 12 as a brake for excessive tissue regeneration acting at the end of the injury and repair process. In Casp1-/- mice there was also at day 5 an enhanced infiltration of inflammatory cells (macrophage, CD4+ cells and neutrophils) in the lamina propria, and increased translocation of commensal bacteria in the colonic stroma as measured by a 16S rRNA qPCR assay. The NF-κB pathway, as measured by the total colonic expression of its target gene IkBa, was also hyperactivated. Exogenous administration of IL-18, a final product of caspase 1 activity, completely reversed the Casp1-/- mice susceptibility to DSS-colitis, suggesting that IL-18 is the pivotal mediator of caspase-1 dependent epithelial regeneration. IECs seemed the principal source of ‘reparative’ IL-18, as adoptive transfer of wild type myeloid cells (normally secreting IL-18) to casp1-/- mice did not reverse DSS-induced colitis as did IL-18 exogenous administration. Moreover, protein expression analysis of different cellular compartments in wild type mice (IECs, macrophages, dendritic cells, and lymphocytes) demonstrated the highest IL-18 production by IECs after 5 d of DSS administration.
An interesting “disease profile” was seen in casp12-/- mice. Even if the recovery, as mentioned above, was ‘clinically’ the same as WT mice, stromal inflammation on day 5 to 8 was enhanced with robust macrophage infiltration and increased expression of several NF-κB gene targets and inflammatory cytokines (COX2, Bcl-xl, cyclin D1, IL-1, IL-6, TNFa, MCP-1, Il11, ccl7 and TNFa-induced protein 2), thus confirming a role of caspase 12 in limiting stromal inflammation. Moreover, cap12-/- mice were more susceptible than WT mice to colitis induced by chronic low doses of DSS, which is driven by a sustained inflammation in the lamina propria rather than by a direct epithelial injury.
Both Caspase 1 and caspase 12 deficiency also rendered the mice more prone to tumorigenesis in the DSS+AOM model, suggesting how crucial the uncontrolled chronic inflammation is for colorectal carcinogenesis.
Even though the findings were reminiscent of Rakoff-Nahoum model, authors did not investigate possible connections between TLR/MyD88 and caspase 1/IL-18 signals. TLR4, apart from NF-κB, can activate caspase 1 via the intracellular mediator TRIF[40], and MyD88, as mentioned, regulates also the IL-18 receptor signal. Interaction between MyD88 and caspase 1 activity has been reported by other authors[41]. Moreover, experimental manipulations applied were not consistent across the two studies. For example, the contribution of commensal flora to caspase-1/IL-18 driven repair was not assessed as no test with antibiotics was performed.
IL-18/IL-18R/MYD88 axis: The third key research was published by Salcedo et al[42]. They assessed whether MyD88 deficiency was also associated with increased tumorigenesis by maintaining alive with hydration MyD88-/- mice after the DSS course, and then administering AOM to recapitulate the pathogenesis of CAC. Authors confirmed a much higher mean number of colonic polyps in Myd88 -/- than in WT mice at day 60 after AOM challenge, thus suggesting that the pro-inflammatory state of these mice enhances also the tumorigenic effect of AOM.
It was shown, however, by labelling test for Ki67, that epithelial proliferation and regeneration were impeded in Myd88-/- mice, a feature that is difficult to reconcile with the increased susceptibility to the development of polyps after AOM/DSS treatment. To clarify this aspect, authors performed a gene expression profiling of the total colons of AOM/DSS-treated Myd88-/- and WT mice and looked at the expression of cell proliferation-associated genes. They found that genes of the epidermal growth factor receptor, Met, and beta-catenin pathways were preferentially induced in Myd88-/- mice after AOM/DSS administration, indicating that compensatory mechanisms are apparently activated in the abortive attempt to maintain the integrity of the colonic mucosa. Such an ‘abortive attempt’ would therefore fail in repairing the tissue while rendering the mice more sensitive to the tumorigenic action of AOM. Moreover a decreased expression of mismatch DNA repair genes was also observed, which correlated with a higher frequency of beta-catenin mutations and colon adenocarcinomas formation after a prolonged period of observation. In parallel IL-18 -/- and IL-18 receptor (IL-18R) -/- mice were also investigated, observing a similar phenotype, although attenuated, to MyD88-/-. Moreover, similar gene expression profiles were seen for IL-18-/- and IL-18R-/- mice. The precise connection between MyD88 and IL-18 was still not investigated, and Salcedo et al[42] concluded that the more probable explanation of such an interplay would rely on the permissive role of MyD88 upon IL-18R activation, which allows for epithelial regeneration. As mentioned above, a possible MyD88-dependent production of IL-18 cannot be excluded[43]. Moreover, it was not clarified whether this MyD88/IL-18 circuit that implies self-regulation of epithelial cell growth is completely ‘epithelium confined’ (autocrine loop) or involvement of stromal components is required. Experienced researchers argue that an involvement of stromal macrophages is necessary in the reparative process, so that IL-18 is released by IECs upon injury and activates underlying IL18-R-expressing macrophages which in turn produce epithelial growth factors targeting IECs for the regenerative task[44]. Nonetheless, a completely autocrine circuit cannot be excluded since IECs express IL-18R[45], and it has been demonstrated that stromal infiltration by myeloid cells does not influence the DSS-induced colitis in the casp1-/- model.
Overall these three studies highlight the dual role of innate immunity. The baseline natural immune response to commensal microbes is mainly epithelium-confined, is necessary to protect against inflammation and cancer not only for its bactericidal effect but also for a fine regulation of epithelial cell cycle, and alteration with antibiotics of the intestinal ecology is a cancer predisposing factor. On the other hand, the same innate immunity pathways are responsible for cancer development in chronic colitis thorough maintenance of an “inflammatory vicious circle” which is mainly stromal-based.
Apart from caspase 12, the “inflammatory brake” investigated in the study by Dupaul-Chicoine et al[34] other two studies have explored molecules known for dampening the inflammatory response.
SIGIRR: In a genetic engineered mouse model by Xiao et al[46] the effect of the inhibitory SIGIRR (Single immunoglobulin IL-1-related receptor) molecule was investigated for relation to colitis and CAC. SIGIRR is an intracellular inhibitor of the TLRs pathway, yet SIGIRR -/- mice have an higher susceptibility to DSS similar to TLR4 and TLR2 -/- mice, suggesting that SIGIRR deficiency may contribute to enhanced DSS-colitis with a mechanism different from the impaired epithelial homeostasis seen for TLR4-/-, TLR2-/- and MyD88-/- models.
It was suggested that epithelial SIGIRR functions as regulator of normal immune response to commensal microbes and when absent an excessive inflammatory response to normally colonizing bacteria occurs thus determining epithelial disruption and increased severity of DSS-induced colitis and AOM+DSS tumorigenesis.
However SIGIRR is also an inhibitor of IL-18/IL-18R axis[47], which has been demonstrated pivotal in epithelial regeneration. On that respect SIGIRR -/- mice should be more protected from, rather than susceptible to, DSS insult, but this was not the case of Xiao’s model. Effect of SIGIRR deficiency on IL-18 function was not investigated by Xiao et al[46].
NLRP6: The second investigated ‘inflammatory brake’ was a member of NLR family containing a pyrin domain, the NLRP6. Some NRLs (e.g., NOD1 and NOD2) induce anti-microbial cytokines expression via NF-κB and MAPK signalling, whereas others (e.g., NLRP1, NLRP3 and NLRC4) trigger inflammatory caspases and formation of inflammasome protein complexes[48]. NLRP6 has only recently been characterized and seems to down-regulate inflammatory response in the intestinum[49] thus preventing chronic colitis and subsequent tumorigenesis.
In a recent research by Normand et al[50], a mouse model of genetic NLRP6 deficiency (nlrp6 -/-) was used to demonstrate that this receptor is mainly expressed by fibroblast within the colonic mucosa and its absence is associated with enhanced and prolonged inflammatory reaction following DSS administration for 7 d. Mice had also worse surrogate-markers of colitis as compared to WT animals, such as weight loss, rectal bleeding and diarrhoea. Moreover NLRP6 deficiency was also associated to superior tumorigenesis in the DSS + AOM experiment.
Overall, these studies exploring knockout models for ‘brakes’ of inflammation confirm that all molecular components (negative or positive regulators) are fundamental for the fine epithelial homeostasis and cancer prevention. Altering in any direction pro-inflammatory and/or anti-inflammatory mediators leads to the same pro-colitic/pro-cancerogenic effect.
The IL-23/T helper 17 cells (Th17) pathway is recognized as one of the most important etiological factors in IBD and CRC development and studies evaluating the pro-inflammatory or anti-inflammatory roles of IL-17, the distinctive cytokine of Th17, are ongoing.
Th17 are a subset of T helper cells producing IL-17 discovered in 2007. They are considered developmentally distinct from Th1 and Th2 cells and excessive amount of these cells is thought to play a key role in autoimmune pathologies such as Crohn’s disease[51].
The physiological role of Th17 cells is suggested by studies that have demonstrated preferential induction of IL-17 in cases of host infection with various bacterial and fungal species at the epithelial/mucosa interface. Among the cytokines released by Th17 is also IL-22 which stimulates epithelial cells to produce anti-microbial proteins specifically directed against certain types of microorganisms such as Candida spp and Staphylococcus spp. A lack of Th17 cells has been demonstrated to render the host more susceptible to opportunistic infections[52].
The process of Th17 maturation and differentiation from naive T cells is not yet completely understood. A combination of cytokines, including transforming growth factor (TGF)-β, IL-6, IL-1, IL-21 and IL-23 have been implicated, whereas IFNγ and IL-4, the main stimulators of Th1 and Th2 differentiation, respectively, have been shown to inhibit Th17 differentiation[53]. IL-23, however, is thought to be the main stimulator of IL-17 production by Th17 and other IL-17 producing cells[54].
Gene expression signature compatible with Th17 cell infiltration has been associated with shorter disease free survival in patients with radically resected stage I/II colorectal cancer, and this was explained by the immunosuppressive role of Th17 cells[55].
Grivennikov et al[56] confirmed a nearly three-fold higher expression of IL-23 and IL-17 in tumor tissue of 7 colorectal cancer patients as compared to the adjacent normal mucosa. They also studied the role of IL-23 and IL-17 in a mouse model of colorectal carcinogenesis based on the preferential allelic loss of Apc in the colonic epithelium (the so-called CPC-APC mice). CPC-APC mice reproduce human colorectal cancer development in that preneoplastic lesions may be indentified (dysplasia) that progress to overt adenocarcinoma in the distal colon. IL-23 and IL-17 were upregulated also in CRC of CPC-APC mice and this upregulation was seen at early phases of tumor progression. Flow citrometry analysis demonstrated that IL-23 producing cells within the tumor were mainly CD11b+ and F4/80+ myeloid cells infiltrating the stroma.
In CPC-APC mice knockout for IL-23, both number and size of spontaneously developing CRCs were reduced, as were IL-23-induced cytokines (IL-17, IL-6 and IL-22) and epithelial STAT3 phosphorylation, a mediator of epithelial proliferation. The same effect was seen in CPC-APC chimeras mice where IL-23 was specifically knocked-out in bone marrow-cells confirming that this cell type generates the tumorigenic IL-23 signalling. Similar effect was seen in CPC-APC mice knockout for the receptor of IL-17.
Basal IL-23 intestinal production is thought to be maintained by commensal flora and signalling through the innate immunity pathway TLRs and MyD88[57]. As for other mouse models[58], also CPC-APC mice with ablated MyD88 had reduced IL-23 and IL-17 production with reduced spontaneous tumor load, thus indicating the importance of innate immunity signalling for the IL-23/Th17 pathway. This effect was prevented by bone marrow transplantation of MyD88 competent cells. Broad-sprectum antibiotics also reduced IL-23 colonic synthesis and spontaneous tumorigenesis in CPC-APC mice.
Grivennikov et al[56] also demonstrated that preneoplastic lesions in CPC-APC mice are associated with altered mucin production and epithelial barrier homeostasis, which favour commensal bacterial translocation with activation of lamina propria myeloid cells to produce IL-23. Authors postulated that, following an initial cancerogenic mutation, integrity of epithelial barrier in colonic adenomas is lost and stimulation of underlying myeloid cells by translocated commensals is the promoting loop that ultimately leads to adenocarcinoma growth and progression.
A relationship between IL-17 and CRC formation was also identified using another spontaneous intestinal tumorigenesis mouse model (mice bearing a heterozygote mutation of APC: ApcMin/+ mice) targeting the small intestine. Specifically, it was reported that enterotoxigenic Bacteroides fragilis, a human intestinal commensal bacteria, promoted intestinal tumor formation and that tumor formation was inhibited when the IL-17 or its receptor were blocked[59,60].
In a recent study by Hyun et al[61], it was also demonstrated a role for IL-17 in the tumor initiation stage of CAC development, using the DSS+AOM experiment in both wild type and IL17-/- mice. Indeed, the ablation of IL-17 signalling significantly decreased the expression of IL-6, STAT3, TNF-alpha and IFN-gamma in the IL17-/- group, indicating a significant change in the environment driven by this cytokine upon DSS+AOM administration. The degree of epithelial proliferation observed after hematoxylin and eosin staining was also significantly reduced in the IL17-/- group. Furthermore, Ki-67 immunohistochemical staining was performed, and the number of stained cells was found to be significantly decreased in both normal intestinal crypts and tumor specimens. This reduction correlated with decreased b-catenin, cyclin D1, cyclin-dependent kinase 2 and cyclin E expression, suggesting a prominent role of IL-23/Th17 pathway at early stages of tumor development.
Once a CRC is established, a number of immune cell types may differentially contribute to its progression or its growth control (Table 1).
| Immune cell type: phenotype and description | Effect on colorectal cancer prognosis |
| CD8+CD45RO+ T cells: memory cytotoxic T cells, associated with a T helper 1 orientation | Favorably influencing relapse-free, progression-free and overall survival |
| FOXP3+CD25+CD4+ T cells: Regulatory T cells with suppressive effect on effector cytotoxic lymphocytes | Unfavorably influencing relapse-free and overall survival |
| CD14+CD163-IL10high M1 macrophages: classically activated macrophages | Favorably influencing prognosis by enhancing Th1-type antitumor immune response |
| CD14+CD163+IL12high M1 macrophages: classically activated macrophages | Unfavorably influencing prognosis by exerting immunosuppressive effect and enhancing Th2-type response |
| Lin-HL-ADR-CD11b+CD33+ cells: Myeloid-derived suppressor cells | Unfavorably influencing prognosis by promoting metastatic spread |
A wealth of evidence has documented the favorable effect of infiltrating cytotoxic lymphocytes (adaptive immunity) on prognosis for many human cancer types[62-72].
Identification of reliable immune prognostic factors for colorectal cancer patients is the focus of intensive clinical and translational research, and a cornerstone study was published by Pagès et al[73] in 2009. They demonstrated that the combined immunohistochemical analysis of CD8+ and CD45RO+ cells in specific tumor regions may be predictive of disease recurrence and overall survival in patients with early-stage, radically resected colorectal cancer.
First, authors demonstrated an upregulation of gene clusters referring to CD8 cytotoxicity and Th1 orientation in 15 tumors with high density of memory CD45RO+ cells at the immunohistochemistry, and in particular an upregulation of CD8, granzyme, perforin, T-bet, interferon-gamma, interleukin 12Rb 1 and 2, and IL-18 genes. They then demonstrated, in a cohort of 411 colorectal cancer patients with apparently good prognosis (all stage I or II), that low density of both CD8+ and CD45RO+ was associated with a six-fold to seven-fold increased risk of death. In the multivariable analysis, infiltration of CD8+ and/or CD45RO+ cells was an independent prognosticator together with pT stage and clinical presentation with bowel perforation. These results were further confirmed in an independent cohort of 191 patients (validation cohort). In another dataset by the same authors[74], degree of cytotoxic CD8-positive and memory CD45RO-positive T cell infiltration at the primary tumor site (the so-called immune score, Im) displayed a discriminatory prognostic power superior to that of standard staging system and patients with high Im had a significantly prolonged disease-free and overall survival. The Im ranged from 0 to 4 and was based on the assignment of 1 point for the presence of high density of either CD8+ or CD45RO+ cells evaluated at both the central zone and the invasive margins of the primary tumor. In a sample of 415 patients, proportions of cases falling in Im0, Im1, Im2, Im3 and Im4 classes were 9%, 12%, 27%, 22%, and 30%, respectively. The 5 year disease free survival rate was 85%, 53% and 32% for Im4, Im1 and Im0, respectively, with statistically significant P-value. Immune cell infiltration correlated significantly with tumor stage, in particular a decrease of 50% in CD8+ cell density was seen between stage I and IV. Same authors documented, in a separate study, that effector T cells prevented from more aggressive tumor behavior such as early lymphovascular embolisation[75].
We also confirmed the prognostic value of memory CD8+CD45RO+ cells in metastatic patients[76] by evaluating peripheral expression of CD45RO, PD-1, and TLR4 immune pathways in metastatic CRC. We enrolled 31 patients in a prospective study which included a standard firstline chemotherapy with fluorouracil, irinotecan and bevacizumab (clinical trial.gov NCT01533740). Blood was drawn before the first and third cycle of chemotherapy and analyzed by flow cytometry for frequency of CD4+, CD8+, CD45RO+, and PD1+ mononuclear cells and for TLR4 expression on neutrophils.
Two cycles of chemotherapy determined changes in immune variables that were prognostically meaningful. Pre-third-cycle CD45RO+CD8+cell frequency displayed a statistically significant association with progression-free survival (PFS) (median PFS 22.4 vs 9.4 mo for patients with CD45RO+CD8+cell frequency > vs < the median value of 12%) and overall survival (2-year OS rate 62 vs 44%, respectively). A Cox regression multivariate analysis for PFS including pre-third-cycle CD45RO+CD8+cell frequency, CEA, LDH, and Köhne risk class demonstrated CD45RO+CD8+cell frequency to be the only independent prognostic factor.
The beneficial effect of Th1 orientation has also been demonstrated for CAC. A recent study have shown that, using another well-established experimental model of oral AOM+ intrarectal administration of trinitrobenzene sulfonic acid, mice deficient in INF-gamma develop significantly more neoplasms compared to wt mice and Th2-biased IL4-/- mice[77]. On the basis of these results, authors suggest that a Th2-dominant cytokine response may enhance CRC growth in a colitic background. Furthermore, Th2-related cytokines, such as IL-4 and IL-13, determined the upregulation of activation-induced cytidine deaminase (AID), an enzyme which induces DNA mutation in cultured colonic epithelial cells thus favoring accumulation of genetic lesions leading to cancer progression[78].
Still Bindea et al[79] have recently studied 28 immune cell subtypes (the immunome) at all stages of CRC development and found that during cancer progression there is an increasing disappearing within the tumor tissue of cytotoxic T cells with B cells and T follicular helper cells becoming more prevalent. This immune cell shift is probably driven by an enhanced CXCL13/IL-21 signal.
As a general consideration, these results demonstrated that an adaptive, cancer-specific, immune response exists both for localized and metastatic cancer. This immune defence is mainly cytotoxic Th1-oriented and even if it fails to determine a complete “cancer clearance” may still control the occurrence of metastasis and delay disease progression.
Tregs are a subset of CD4+ cells, whose frequency ranges between 4%-10%, that are found to potently restrain specific immune responses and maintain self-tolerance thus preventing autoimmune disorders[80].
Tregs have been implicated in cancer progression as they are able to inhibit antigen specific T cell antitumor response and high peripheral and tumor tissue levels of these cells have been found in colorectal cancer patients[81].
Indeed, specific immune T cell response to tumor associated antigens frequently occurs in cancer patients[82,83], yet effective immune-mediated tumor clearance is uncommon and Tregs seems to play a major role in this context.
In patients where immune constraint is less prominent, T cells infiltrate tumor tissue and this is a well-recognized favourable prognostic sign[84]. In particular, a high ratio of infiltrating CD3+ cells (effector cells) to FOXP3+ cells (Tregs) is associated with improved disease free-survival[85]. Furthermore, depletion of circulating Tregs in mouse models, even for a limited period of time, is associated with rejection of both tumor transplants and de novo chemically-induced neoplasms[86-88]. Treg generation in colorectal cancer appears to be antigen-specific[89].
In a study by Betts et al[90], peripheral and tumor tissue lymphocytes from nearly 50 patients with localized colorectal cancer were immunophenotyped before and at 12 and 52 wk after radical resection and compared with age-matched healthy controls. Before surgery, a nearly 20% increase in FOXP3 content of peripheral Tregs from colorectal cancer patients was observed as compared to Tregs from controls. As FOXP3 expression correlates with the suppressive capacity of these cells[91], the finding indicates that function of Tregs, more than frequency, is enhanced in colorectal cancer patients. In the tumor tissue, Tregs were even more represented than in the periphery, as FOXP3+ cell percentage within the CD4+ pool shifted from nearly 15% in the bloodstream to 50% in the tumor stroma.
It is possible that the enhanced Treg homing to intestinal mucosa seen in cancer patients could be the results of increased expression of certain integrins and adhesion molecules, e.g., CD49d. Same significant difference of FOXP3+ cell percentage between bloodstream and peripheral tissues were reported by Deng et al[92] who compared PBMCs to lymphocytes from the tumor draining lymphnodes.
Even more intriguing, peripheral Treg FOXP3 expression significantly dropped immediately after surgery and, after 52 wk of follow up, it became comparable to that of healthy controls. Finally, specific immune response to certain tumor antigens (e.g., 5T4) were suppressed preoperatively (as measured by ELIspot test of CD4+ cells producing IFN-gamma before and after Tregs depletion) and restored post-operatively, at 6 mo, because of a reduced suppressive Treg action. Moreover, higher Treg-mediated immunosuppression (as measured by ELIspot reaction to tumor antigens CEA and 5T4 after Treg depletion) at the pre-operative timepoint was observed in 10 patients who relapsed as compared to 34 who did not relapse after one year.
Action of Tregs in colorectal cancer may partly explain disappointing results of anticancer vaccination in this disease[93].
In other datasets, however, increased number of tumor infilitrating Tregs has been seen to correlate with improved prognosis. Suggestions have been made on the possible favourable role of Tregs in suppressing protumoral inflammatory response in these cases[94,95].
Important players in CRC are tumor-associated macrophages, which can be divided into classically activated macrophages (M1), typically induced by IFN-gamma and LPS, and alternatively activated macrophages (or M2), induced by IL-4 and IL-13. M1 exhibit potent microbicidal properties and promote strong IL-12-mediated Th1 responses, whilst M2 support Th2-associated functions[96]. M1 macrophages can prime anti-tumor immune response through the production of cytokines like TNF-alpha, IL-6 and IL-12, while M2 can inhibit ant-cancer immunity through the production of TGF-β and IL-10. The balance between M1 and M2 may condition towards a protumoral or antitumoral milieu[97,98].
Recent studies have identified myeloid-origin cells as potent suppressors of tumor immunity and therefore a significant impediment to cancer immunotherapy. “Myeloid-derived suppressor cells” (MDSC), a heterogeneous population of early myeloid progenitors, immature granulocytes, macrophages, and dendritic cells at different stages of differentiation, accumulate in the bloodstream, lymph nodes, bone marrow and at the tumor site of most patients and animals and inhibit both adaptive and innate immunity. MDSC are induced by tumor-secreted and host-secreted factors, especially proinflammatory molecules. The induction of MDSC by proinflammatory mediators led to the hypothesis that inflammation promotes the accumulation of MDSC that down-regulate adaptive immune surveillance and antitumor immunity, thereby facilitating tumor growth[99].
Relatively little is known about neutrophils in human cancers. Several cytokines secreted from both tumor and stromal cells seem to contribute to high neutrophil count (neutrophilia) seen in many metastatic CRC patients and neutrophilia appears to have immunosuppressive consequences. Among others, main neutrophilia-inducing cytokines are vascular endothelial growth factor-A, IL1-beta and IL-6[100]. Neutrophils usually are able to directly phagocyte invading microorganisms (such as bacteria and fungi) or kill them by releasing activating cytokines (TNF-α, IL-1 and interferons), defensins and toxic substances such as reactive oxygen species. Although neutrophils are traditionally considered for their antibacterial functions, it is becoming clear that tumor-associated neutrophils (TAN), peripheral neutrophils and granulocytic myeloid-derived suppressor cells observed in the spleen, bone marrow and bloodstream play an important role in cancer biology, mainly as immunosuppressive cells in the context of tumors[101]. More recently, it has been demonstrated that in untreated tumors, neutrophils can also assume a pro-tumorigenic state, which by analogy to macrophages, is called “N2” phenotype. The full range of mechanisms responsible for this pro-tumorigenic activity have not yet been elucidated, but neutrophils are known to impact angiogenesis, immune surveillance, as well as to secrete chemokines, cytokines and reactive oxygen species. However, it has been noted that under certain conditions (e.g., after TGF-β blockade), TAN can take on an “N1” phenotype, which is pro-inflammatory and antitumorigenic. It is important to consider which of these two possible phenotypes predominate when interpreting the nature of inflammation during CRC development. Interestingly, recent findings have been introduced the peripheral neutrophil to lymphocyte ratio as an important prognostic and predictive factor in CRC[102,103].
Overall a great amount of research has been produced on the immune response in colorectal cancer.
Many assumptions, such as the detrimental effect of inflammatory pathway activation, have partly been called into question since it may be paramount in maintaining epithelial homeostasis. Immune and inflammatory response to CRC is incredibly complex and adapt according to carcinogenetic phase (preneoplastic lesion vs overt carcinoma), cancer stage and microenvironmental context (commensal ecology).
The key interpretation of published results is that a fine balance exists between immune and epithelial/tumor cells and either excessive or defective deviations from this balance will result in cancerogenic and/or cancer progression risk.
Therapeutic immune interventions have necessarily to be multitargeting and finely tuned. A coordinated approach involving concomitantly potentiation of cytotoxic immunity, suppression of protumoral Tregs, Th17 and Inflammatory cells and reconstitution of the normal host/microbial symbiosis is the only way, to our opinion, to render effective immunotherapy in CRC care.
P- Reviewer: Chen Z, Koutsilieris M, Matsui H S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9831] [Article Influence: 819.3] [Reference Citation Analysis (4)] |
| 2. | Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 1652] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 3. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] |
| 4. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [PubMed] |
| 5. | Ponz de Leon M, Di Gregorio C. Pathology of colorectal cancer. Dig Liver Dis. 2001;33:372-388. [PubMed] |
| 6. | Monteleone G, Pallone F, Stolfi C. The dual role of inflammation in colon carcinogenesis. Int J Mol Sci. 2012;13:11071-11084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854-862. [PubMed] |
| 8. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [PubMed] |
| 9. | Ferrone C, Dranoff G. Dual roles for immunity in gastrointestinal cancers. J Clin Oncol. 2010;28:4045-4051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 10. | Tjalsma H, Boleij A, Marchesi JR, Dutilh BE. A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol. 2012;10:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 620] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 11. | McCOY WC, MASON JM. Enterococcal endocarditis associated with carcinoma of the sigmoid; report of a case. J Med Assoc State Ala. 1951;21:162-166. [PubMed] |
| 12. | Boleij A, van Gelder MM, Swinkels DW, Tjalsma H. Clinical Importance of Streptococcus gallolyticus infection among colorectal cancer patients: systematic review and meta-analysis. Clin Infect Dis. 2011;53:870-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 13. | Corredoira-Sánchez J, García-Garrote F, Rabuñal R, López-Roses L, García-País MJ, Castro E, González-Soler R, Coira A, Pita J, López-Álvarez MJ. Association between bacteremia due to Streptococcus gallolyticus subsp. gallolyticus (Streptococcus bovis I) and colorectal neoplasia: a case-control study. Clin Infect Dis. 2012;55:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009;69:4918-4925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 717] [Article Influence: 44.8] [Reference Citation Analysis (1)] |
| 15. | Abdulamir AS, Hafidh RR, Bakar FA. Molecular detection, quantification, and isolation of Streptococcus gallolyticus bacteria colonizing colorectal tumors: inflammation-driven potential of carcinogenesis via IL-1, COX-2, and IL-8. Mol Cancer. 2010;9:249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Biarc J, Nguyen IS, Pini A, Gossé F, Richert S, Thiersé D, Van Dorsselaer A, Leize-Wagner E, Raul F, Klein JP. Carcinogenic properties of proteins with pro-inflammatory activity from Streptococcus infantarius (formerly S.bovis). Carcinogenesis. 2004;25:1477-1484. [PubMed] |
| 17. | Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 917] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 18. | Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183-1188. [PubMed] |
| 19. | Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 649] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 20. | Ivanov NA, Kholodok GN, Kulish ID. [Comparative analysis of antibiotic sensitivity of Streptococcus pneumoniae strains isolated from patients and carriers]. Antibiot Khimioter. 1990;35:32-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Boleij A, Roelofs R, Danne C, Bellais S, Dramsi S, Kato I, Tjalsma H. Selective antibody response to Streptococcus gallolyticus pilus proteins in colorectal cancer patients. Cancer Prev Res (Phila). 2012;5:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Ohkusa T, Yoshida T, Sato N, Watanabe S, Tajiri H, Okayasu I. Commensal bacteria can enter colonic epithelial cells and induce proinflammatory cytokine secretion: a possible pathogenic mechanism of ulcerative colitis. J Med Microbiol. 2009;58:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Nagamine CM, Rogers AB, Fox JG, Schauer DB. Helicobacter hepaticus promotes azoxymethane-initiated colon tumorigenesis in BALB/c-IL10-deficient mice. Int J Cancer. 2008;122:832-838. [PubMed] |
| 24. | Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1374] [Cited by in RCA: 1618] [Article Influence: 124.5] [Reference Citation Analysis (1)] |
| 25. | Fukata M, Arditi M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013;6:451-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 26. | Creagh EM, O’Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352-357. [PubMed] |
| 27. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [PubMed] |
| 28. | Breynaert C, Vermeire S, Rutgeerts P, Van Assche G. Dysplasia and colorectal cancer in inflammatory bowel disease: a result of inflammation or an intrinsic risk? Acta Gastroenterol Belg. 2008;71:367-372. [PubMed] |
| 29. | Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789-799. [PubMed] |
| 30. | Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229-241. [PubMed] |
| 31. | Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089-1097. [PubMed] |
| 32. | Feng T, Elson CO, Cong Y. Microbiota: dual-faceted player in experimental colitis. Gut Microbes. 2010;1:388-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Araki A, Kanai T, Ishikura T, Makita S, Uraushihara K, Iiyama R, Totsuka T, Takeda K, Akira S, Watanabe M. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J Gastroenterol. 2005;40:16-23. [PubMed] |
| 34. | Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 417] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 35. | Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 891] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 36. | Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol. 2007;27:98-114. [PubMed] |
| 37. | Sivakumar PV, Westrich GM, Kanaly S, Garka K, Born TL, Derry JM, Viney JL. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut. 2002;50:812-820. [PubMed] |
| 38. | Zhernakova A, Festen EM, Franke L, Trynka G, van Diemen CC, Monsuur AJ, Bevova M, Nijmeijer RM, van ‘t Slot R, Heijmans R. Genetic analysis of innate immunity in Crohn’s disease and ulcerative colitis identifies two susceptibility loci harboring CARD9 and IL18RAP. Am J Hum Genet. 2008;82:1202-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 39. | Siegmund B. Interleukin-18 in intestinal inflammation: friend and foe? Immunity. 2010;32:300-302. [PubMed] |
| 40. | Tsutsui H, Imamura M, Fujimoto J, Nakanishi K. The TLR4/TRIF-Mediated Activation of NLRP3 Inflammasome Underlies Endotoxin-Induced Liver Injury in Mice. Gastroenterol Res Pract. 2010;2010:641865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Miggin SM, Pålsson-McDermott E, Dunne A, Jefferies C, Pinteaux E, Banahan K, Murphy C, Moynagh P, Yamamoto M, Akira S. NF-kappaB activation by the Toll-IL-1 receptor domain protein MyD88 adapter-like is regulated by caspase-1. Proc Natl Acad Sci USA. 2007;104:3372-3377. [PubMed] |
| 42. | Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, Wang E, Ma W, Haines D, O’hUigin C. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625-1636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 336] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 43. | Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869-1881. [PubMed] |
| 44. | Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol. 2011;11:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 314] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 45. | Kolinska J, Lisa V, Clark JA, Kozakova H, Zakostelecka M, Khailova L, Sinkora M, Kitanovicova A, Dvorak B. Constitutive expression of IL-18 and IL-18R in differentiated IEC-6 cells: effect of TNF-alpha and IFN-gamma treatment. J Interferon Cytokine Res. 2008;28:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Xiao H, Gulen MF, Qin J, Yao J, Bulek K, Kish D, Altuntas CZ, Wald D, Ma C, Zhou H. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461-475. [PubMed] |
| 47. | Riva F, Bonavita E, Barbati E, Muzio M, Mantovani A, Garlanda C. TIR8/SIGIRR is an Interleukin-1 Receptor/Toll Like Receptor Family Member with Regulatory Functions in Inflammation and Immunity. Front Immunol. 2012;3:322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol. 2010;10:688-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 333] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 49. | Anand PK, Malireddi RK, Lukens JR, Vogel P, Bertin J, Lamkanfi M, Kanneganti TD. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature. 2012;488:389-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 320] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 50. | Normand S, Delanoye-Crespin A, Bressenot A, Huot L, Grandjean T, Peyrin-Biroulet L, Lemoine Y, Hot D, Chamaillard M. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc Natl Acad Sci USA. 2011;108:9601-9606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 296] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 51. | Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123-1132. [PubMed] |
| 52. | Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281-286. [PubMed] |
| 53. | Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 987] [Cited by in RCA: 1057] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 54. | McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17-23. [PubMed] |
| 55. | Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 889] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 56. | Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1024] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 57. | Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Bérard M, Kleinschek M, Cua D, Di Santo JP, Eberl G. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 494] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 58. | Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124-127. [PubMed] |
| 59. | Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1113] [Cited by in RCA: 1280] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 60. | Chae WJ, Gibson TF, Zelterman D, Hao L, Henegariu O, Bothwell AL. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc Natl Acad Sci USA. 2010;107:5540-5544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 191] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 61. | Hyun YS, Han DS, Lee AR, Eun CS, Youn J, Kim HY. Role of IL-17A in the development of colitis-associated cancer. Carcinogenesis. 2012;33:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 62. | Eerola AK, Soini Y, Pääkkö P. A high number of tumor-infiltrating lymphocytes are associated with a small tumor size, low tumor stage, and a favorable prognosis in operated small cell lung carcinoma. Clin Cancer Res. 2000;6:1875-1881. [PubMed] |
| 63. | Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10:4450-4456. [PubMed] |
| 64. | de Jong RA, Leffers N, Boezen HM, ten Hoor KA, van der Zee AG, Hollema H, Nijman HW. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 65. | Oshikiri T, Miyamoto M, Shichinohe T, Suzuoki M, Hiraoka K, Nakakubo Y, Shinohara T, Itoh T, Kondo S, Katoh H. Prognostic value of intratumoral CD8+ T lymphocyte in extrahepatic bile duct carcinoma as essential immune response. J Surg Oncol. 2003;84:224-228. [PubMed] |
| 66. | Schumacher K, Haensch W, Röefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932-3936. [PubMed] |
| 67. | Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, Bajorin DF, Reuter VE, Herr H, Old LJ. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA. 2007;104:3967-3972. [PubMed] |
| 68. | Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26-e31. [PubMed] |
| 69. | Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407-414. [PubMed] |
| 70. | Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104:3360-3365. [PubMed] |
| 71. | Donnem T, Al-Shibli K, Andersen S, Al-Saad S, Busund LT, Bremnes RM. Combination of low vascular endothelial growth factor A (VEGF-A)/VEGF receptor 2 expression and high lymphocyte infiltration is a strong and independent favorable prognostic factor in patients with nonsmall cell lung cancer. Cancer. 2010;116:4318-4325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | Anraku M, Cunningham KS, Yun Z, Tsao MS, Zhang L, Keshavjee S, Johnston MR, de Perrot M. Impact of tumor-infiltrating T cells on survival in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2008;135:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 73. | Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944-5951. [PubMed] |
| 74. | Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pagès F. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 770] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 75. | Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654-2666. [PubMed] |
| 76. | Formica V, Cereda V, di Bari MG, Grenga I, Tesauro M, Raffaele P, Ferroni P, Guadagni F, Roselli M. Peripheral CD45RO, PD-1, and TLR4 expression in metastatic colorectal cancer patients treated with bevacizumab, fluorouracil, and irinotecan (FOLFIRI-B). Med Oncol. 2013;30:743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Osawa E, Nakajima A, Fujisawa T, Kawamura YI, Toyama-Sorimachi N, Nakagama H, Dohi T. Predominant T helper type 2-inflammatory responses promote murine colon cancers. Int J Cancer. 2006;118:2232-2236. [PubMed] |
| 78. | Endo Y, Marusawa H, Kou T, Nakase H, Fujii S, Fujimori T, Kinoshita K, Honjo T, Chiba T. Activation-induced cytidine deaminase links between inflammation and the development of colitis-associated colorectal cancers. Gastroenterology. 2008;135:889-898, 898.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 79. | Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1792] [Cited by in RCA: 2853] [Article Influence: 237.8] [Reference Citation Analysis (0)] |
| 80. | Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 565] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 81. | Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, Torkington J, Rees BI, Williams GT, Gallimore AM. CD4+CD25+FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS One. 2006;1:e129. [PubMed] |
| 82. | Campi G, Crosti M, Consogno G, Facchinetti V, Conti-Fine BM, Longhi R, Casorati G, Dellabona P, Protti MP. CD4(+) T cells from healthy subjects and colon cancer patients recognize a carcinoembryonic antigen-specific immunodominant epitope. Cancer Res. 2003;63:8481-8486. [PubMed] |
| 83. | Nagorsen D, Scheibenbogen C, Letsch A, Germer CT, Buhr HJ, Hegewisch-Becker S, Rivoltini L, Thiel E, Keilholz U. T cell responses against tumor associated antigens and prognosis in colorectal cancer patients. J Transl Med. 2005;3:3. [PubMed] |
| 84. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [PubMed] |
| 85. | Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137:1270-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 255] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 86. | Jones E, Dahm-Vicker M, Simon AK, Green A, Powrie F, Cerundolo V, Gallimore A. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun. 2002;2:1. [PubMed] |
| 87. | Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128-3133. [PubMed] |
| 88. | Betts G, Twohig J, Van den Broek M, Sierro S, Godkin A, Gallimore A. The impact of regulatory T cells on carcinogen-induced sarcogenesis. Br J Cancer. 2007;96:1849-1854. [PubMed] |
| 89. | Bonertz A, Weitz J, Pietsch DH, Rahbari NN, Schlude C, Ge Y, Juenger S, Vlodavsky I, Khazaie K, Jaeger D. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest. 2009;119:3311-3321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 90. | Betts G, Jones E, Junaid S, El-Shanawany T, Scurr M, Mizen P, Kumar M, Jones S, Rees B, Williams G. Suppression of tumour-specific CD4⁺ T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut. 2012;61:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 91. | Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1588] [Cited by in RCA: 1790] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 92. | Deng L, Zhang H, Luan Y, Zhang J, Xing Q, Dong S, Wu X, Liu M, Wang S. Accumulation of foxp3+ T regulatory cells in draining lymph nodes correlates with disease progression and immune suppression in colorectal cancer patients. Clin Cancer Res. 2010;16:4105-4112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 93. | Nagorsen D, Thiel E. Clinical and immunologic responses to active specific cancer vaccines in human colorectal cancer. Clin Cancer Res. 2006;12:3064-3069. [PubMed] |
| 94. | Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691-702. [PubMed] |
| 95. | Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 793] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 96. | Sica A. Role of tumour-associated macrophages in cancer-related inflammation. Exp Oncol. 2010;32:153-158. [PubMed] |
| 97. | Mantovani A, Locati M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arterioscler Thromb Vasc Biol. 2013;33:1478-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 98. | Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155-161. [PubMed] |
| 99. | Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499-4506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1269] [Cited by in RCA: 1353] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 100. | Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 471] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 101. | Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 458] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 102. | Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181-184. [PubMed] |
| 103. | Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011;104:1288-1295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 365] [Article Influence: 26.1] [Reference Citation Analysis (0)] |