Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Sep 14, 2014; 20(34): 12283-12291
Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12283
Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12283
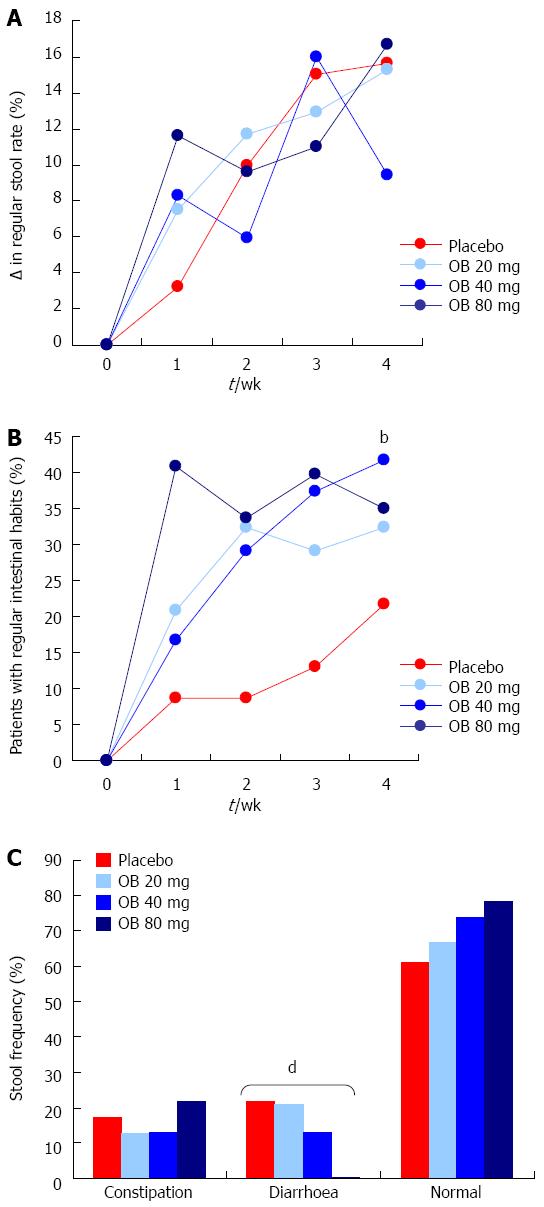
Figure 4 Effect of otilonium bromide (20, 40, 80 mg) and placebo on change in regular stool rate (A), regular intestinal habits (B) and frequency of stool type (C).
Data are presented as % change from baseline values (A + B) or % patients (C). Asterix denotes level of statistical significance (bP < 0.01) for placebo vs 40 mg otilonium bromide at 4 wk (B) and a dose dependent reduction in frequency of diarrhoea (dP < 0.001) (C).
- Citation: Chmielewska-Wilkoń D, Reggiardo G, Egan CG. Otilonium bromide in irritable bowel syndrome: A dose-ranging randomized double-blind placebo-controlled trial. World J Gastroenterol 2014; 20(34): 12283-12291
- URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12283.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12283