Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12330
Revised: February 17, 2014
Accepted: April 28, 2014
Published online: September 14, 2014
Processing time: 260 Days and 0.3 Hours
AIM: To assess the advantages and disadvantages of immunosuppression monotherapy after transplantation and the impact of monotherapy on hepatitis C virus (HCV) recurrence.
METHODS: Articles from Cochrane Hepato-Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded, including non-English literature identified in these databases, were searched up to January 2013. We included randomized clinical trials comparing various immunosuppression monotherapy and prednisone-based immunosuppression combinations for liver transplantation. The modified Jadad scale score or the Oxford quality scoring system was used. Meta-analyses were performed with weighted random-effects models.
RESULTS: A total of 14 randomized articles including 1814 patients were identified. Eight trials including 1214 patients compared tacrolimus monotherapy (n = 610) vs tacrolimus plus steroids or triple therapy regarding acute rejection and adverse events (n = 604). Five trials, including 285 patients, compared tacrolimus monotherapy (n = 143) vs tacrolimus plus steroids or triple therapy regarding hepatitis C recurrence (n = 142). Four trials including 273 patients compared cyclosporine monotherapy (n = 148) vs cyclosporine and steroids regarding acute rejection and adverse events (n = 125). Two trials including 170 patients compared mycophenolate mofetil monotherapy (n = 86) vs combinations regarding acute rejection (n = 84). There were no significant differences in the acute rejection rates between tacrolimus monotherapy (RR = 1.04, P = 0.620), and cyclosporine monotherapy (RR = 0.89, P = 0.770). Mycophenolate mofetil monotherapy had a significant increase in the acute rejection rate (RR = 4.50, P = 0.027). Tacrolimus monotherapy had no significant effects on the recurrence of hepatitis C (RR = 1.03, P = 0.752). More cytomegalovirus infection (RR = 0.48, P = 0.000) and drug-related diabetes mellitus (RR = 0.54, P = 0.000) were observed in the immunosuppression combination therapy groups.
CONCLUSION: Tacrolimus and cyclosporine monotherapy may be as effective as immunosuppression combination therapy. Mycophenolate mofetil monotherapy was not considerable. Tacrolimus monotherapy does not increase recurrence of HCV.
Core tip: Immunosuppression is used to reduce rejection after liver transplantation. There is considerable controversy regarding whether an immunosuppression monotherapy should be used after transplantation. To assess the advantages (lower adverse events such as hypertension) and disadvantages (more episodes of rejection) of immunosuppression monotherapy after transplantation. To assess the impact of immunosuppression monotherapy on recurrence of hepatitis C.
- Citation: Lan X, Liu MG, Chen HX, Liu HM, Zeng W, Wei D, Chen P. Efficacy of immunosuppression monotherapy after liver transplantation: A meta-analysis. World J Gastroenterol 2014; 20(34): 12330-12340
- URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12330.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12330
Liver transplantation (LT) remains the only curative treatment for various liver diseases. Most immunosuppression protocols in liver transplantation currently based on drug combinations provide excellent graft acceptance. However, there are a number of undesired side effects that can dramatically impact patient morbidity. Steroids are associated with many of these complications[1-4]. In addition, the early pretreatment with high-dose steroids may interfere with graft acceptance[5].
Recent investigations have generated biomarkers potentially capable of identifying operationally tolerant liver transplant recipients[6]. Long-term immunosuppression without steroids is not widely practiced in liver transplantation likely due to reports of higher rejection rates with immunosuppression monotherapy[7-11]. Furthermore, there is much debate about the role of steroids and immunosuppression monotherapy on recurrence of hepatitis C virus (HCV)[12-14].
The aim of this study was to conduct a systematic review and meta-analysis to assess the advantages and of an immunosuppression with or without steroids after transplantation.
A detailed set of eligibility criteria (see below, Study Selection) was established a priori to identify studies relevant for this Meta-analysis. A comprehensive search for published and unpublished randomized cohort studies (RCTs) involving adult (≥ 18 years old) subjects who had received immunosuppression combination steroid for recipient post-LT was undertaken. An a priori decision was made to only include reports utilizing immunosuppression monotherapy vs combination therapy which is widely regarded as the current standard of care. There were no restrictions on language placed during our search process.
An electronic search of the following databases was performed: MEDLINE, EMBASE, and the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, and the Science Citation Index Expanded. There were no restrictions on language placed during our search process and all was up to January 2013. The search strategy combined the following key words “randomized or randomize or random”, “liver or hepatic”, “transplantation or transplant or graft”, “hepatitis C or hep C or HCV”, “immunosuppression or immunodepressant or immunosuppressant or immunosuppression agent”, and “monotherapy”. Reference lists of retrieved articles and other reviews were screened to identify other potential studies. When necessary, we attempted to contact researchers to identify missing data not included in the original publication.
Titles and abstracts identified from the search selection process were independently screened by two authors (Xiang Lan and MG Liu). Pilot testing of the 30 initial citations was employed to ensure explicit and valid screening criteria and good inter-observer agreement. All full text articles from potentially eligible studies were then retrieved and independently reviewed by two authors (Xiang Lan and MG Liu) using strict inclusion and exclusion criteria. We included RCTs published in full-text or abstract form comparing combination therapy (including triple therapy: two immunosuppression plus steroid) with the immunosuppression monotherapy (one main immunosuppression and steroid withdraw). This combination therapy is widely regarded as the current standard of care. Studies reporting HCV recurrence must have HCV RNA levels monitoring. Studies had to report on the primary outcome (acute rejection rate), and data on the immunosuppressive regimen post transplantation had to be clearly stated. Besides, the strategy of steroid withdrawing must be stated too. We excluded: (1) companion reports (study with fewer patients and/or shorter follow up excluded); (2) abstracts identified with electronic search published before 1990; (3) review articles, editorials, and case reports; (4) small studies or case series with less than 10 patients on each group; (5) studies on non-LT patient populations or following multi-organ transplan; (6) studies that only included patients re-transplantation; (7) studies in which not all treated patients received combination therapy or therapy was used in conjunction with other/experimental interventions/treatments; and (8) studies reporting strictly on highly selected patient populations only. Where more than one exclusion criteria existed, the primary reason was selected based on the first exclusion criteria encountered as numbered above. If no consensus was reached, a third reviewer (Ping Chen), who was unaware of the other reviewers’ determinations, functioned as an arbitrator.
The methodological quality of each trial was assessed according to the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention[15] and the Cochrane Hepato-biliary Group Module[16]. The modified Jadad scale score or the Oxford quality scoring system[17] were used to independently assess the methodological quality of RCTs. One-3 points indicated low quality. Discrepancies were resolved by re-reviewing the original publications, discussion and reaching consensus. If similar articles in different journals had the same author, duplicate cases were eliminated.
The following data were extracted from each study using a predefined form: year of publication, number of patients, immunosuppression regimen, immunosuppression concentration target, drug dosage, mean immunosuppression after LT, The incidence of acute cellular rejection (ACR) rates, methodological quality, HCV recurrence and other adverse events such as hypertension and diabetes. The evaluated outcome was biopsy-proven ACR triggered by clinical suspicion or by protocol. The risk of bias in eligible studies was assessed by a single reviewer (HX Chen), and checked by a second reviewer (HM Liu) in terms of the quality of selective outcomes. We determined whether groups were balanced at baseline, and whether an intension to treat analysis was undertaken.
The number of events and patients in each intervention arm were used to calculate relative risks (RR) and 95%CI. Heterogeneity between the studies was assessed with the Q statistic, and meta-analyses were performed with weighted random-effects models because of the presence of heterogeneity between studies. Comparability of the studies included in each pooled analysis was confirmed by examination of the χ2Q (expressed as a P value) and I2 statistics of heterogeneity. Statistical heterogeneity was present, defined as P≤ 0.05 or I2 > 50%. If P value was below 0.1 or I2 > 25%, a meta-regression was performed to assess the potential risk of heterogeneity. To account for potential differences that were evident clinically, but not identified by statistical tests, random effects models were used for each outcome measure. All statistical analyses were performed using STATA 10. We planned to perform funnel plots for assessment of risk of bias, but were not able to do so because there were no more than 10 articles in any ananlysis.
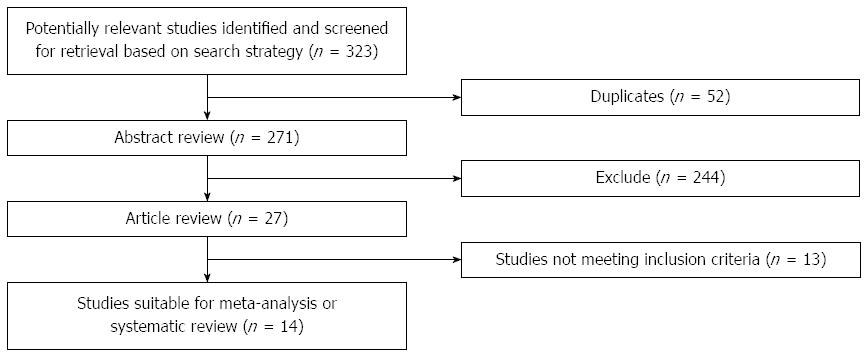
Results of the article selection are described in Figure 1. The initial search from the electronic database yielded a total of 287 abstracts in MEDLINE, 21 in EMBASE and 15 in Cochrane Central Register of Controlled Trials. Fifty-two citations were duplications of identical citations resulting from searching different data bases. After applying limits, 27 study abstracts of RCTs remained. We then excluded 13 citations after title and abstract screening, as they clearly did not fulfill our eligibility criteria. Finally, publications eligible for analysis included 14 RCTs.
Eight trials including 1214 patients compared tacrolimus monotherapy (n = 610) vs tacrolimus plus steroids or immunosuppression triple therapy regarding acute rejection and adverse events (n = 604). Five trials, including 285 patients, compared tacrolimus monotherapy (n = 143) vs tacrolimus plus steroids or immunosuppression triple therapy effects on hepatitis C recurrence (n = 142). Four trials including 273 patients compared cyclosporine monotherapy (148) vs cyclosporine and steroids regarding acute rejection and adverse events (n = 125). Two trials including 170 patients compared mycophenolate mofetil monotherapy (MMF, n = 86) vs combination regarding acute rejection (n = 84). Four studies were based in the Germany, 3 in Italy, Spain and United Kingdom, and one each in United States and France. Details of these studies are shown in Table 1.
| Ref. | Year | Location | Immunosuppression | Number of patients | Drug whole-blood trough levels (ng/mL) | Steroid protocol (POT) | ||
| Monotherapy group | Combination group | Monotherapy group | Combination group | |||||
| Benítez et al[18] | 2010 | Spain | Tacrolimus | Tacrolimus plus prednisone | 37 | 5–12 | 10-15 (3 mo POT) | 20 mg/d prednisone |
| 7-12 thereafter | NS in monotherapy group | |||||||
| Boillot et al[19] | 2005 | Germany | Tacrolimus monotherapy | Tacrolimus plus steroids | 698 | 10-20 (first 6 wk) and 5-15 thereafter | prednisone 1-20 mg/d during month 1, 10-15 mg/day during month 2, and 5-10 mg/d during month 3 and thereafter and NS in monotherapy group | |
| Weiler et al[20] | 2010 | Germany | Tacrolimus monotherapy | Tacrolimus plus steroids | 110 | 10-15 (first 6 wk) and 5-10 thereafter | 100 mg on day 1 to 12 mg on day 14 in both groups and placebo in monotherapy group 2 wk later | |
| Chau et al[21] | 2001 | Great Britain | Tacrolimus monotherapy | Triple therapy | 24 | 5-15 | NS | prednisone 1 mg/kg per day and NS in monotherapy group |
| Moench et al[22] | 2007 | Germany | Tacrolimus monotherapy | Tacrolimus plus steroids | 110 | 10-15 (between day 0 and 42)5-10 thereafter | Methylprednisolon 100 mg at day 1, 75 mg at day 2, 48 mg at day 3 and 4, 36 mg at day 5 and 6, 24 mg at day 7 and 8, 16 mg from day 9 to 13 and 12 mg at day 14 and then placebo in monotherapy group 2 wk later | |
| Eason et al[23] | 2003 | United States | Tacrolimus monotherapy + ATG | Tacrolimus plus steroids | 119 | 10-12 | NS | NS |
| Samonakis et al[12] | 2006 | Great Britain | Tacrolimus monotherapy | Tacrolimus, azathioprine, and prednisolone | 56 | 5-14 | Methylprednisolone(16 mg/d IV) until oral intake was establishedwhen 20 mg/d prednisolone and NS in monotherapy group | |
| Margarit et al[13] | 2005 | Spain | Tacrolimus monotherapy | Tacrolimus plus steroids | 60 | 10-15 over the first few weeks and between 8 and 12 thereafter | 100 mg b.i.d. of methylprednisolone post-LT day 1 and decreasing to 20 mg/d by day 6 patients were weaned off prednisone, if possible, within 3 mo post-LT and NS in monotherapy group | |
| Manousou et al[14] | 2009 | GreatBritain | Tacrolimus monotherapy | Tacrolimus, azathioprine, and prednisolone | 103 | 3-26 over the first few week8 at 1 mo7 at 2 mo8 at 3 mo | 2.5-14 over the first few weeks8.4 at 1 mo7.5 at 2 mo7 at 3 mo | Methylprednisolone (16 mg/d intravenously) was given until oral intake was established; then, 20 mg/d prednisolone and NS in monotherapy group |
| Belli et al[24] | 1998 | Italy | Cyclosporine monotherapy | Cyclosporine plus steroids | 88 | 200-300 (1 mo POT) | Methylprednisolone | |
| 150-250 thereafter | at a dose of 200 mg/d on postoperative day 1, and tapered to 20 mg/d of prednisone by postoperative day 6; 5 mg reductions every 2 wk) to a maintenance dose of 0.1 mg/kg per day and withdraw in monotherapy group 90 d later | |||||||
| De Carlis et al[25] | 1997 | Italy | Cyclosporine monotherapy | Cyclosporine plus steroids | 100 | 200-300 (1 mo POT)150-250 thereafter | Continued with methylprednisolone at a dose of 200 mg/d on postoperative day 1, and tapered to 20 mg/d of prednisone by postoperative day 6; 5 mg reductions every 2 wk) to a maintenance dose of 0.1 mg/kg per day and withdraw in monotherapy group 3 mo later | |
| Chau et al[21] | 2001 | Great Britain | Cyclosporine monotherapy | Triple therapy | 26 | 100-300 | Prednisone 1 mg/kg per day and NS in monotherapy group | |
| Romani et al[26] | 1994 | Italy | Cyclosporine monotherapy | Cyclosporine plus steroids | 59 | 200-300 (1 mo POT) | Methylprednisolone | |
| 150-250 thereafter | at a dose of 200 mg/d on postoperative day 1 and 5 mg reductions every 2 wk) to a maintenance dose of 0.1 mg/kg per day and withdraw in monotherapy group 90 d later | |||||||
| Schlitt et al[27] | 2001 | Germany | Mycophenolate Mofetil Monotherapy | Mycophenolate Mofetil plus CNIs | 28 | 500 mg/d in the first week; 1000 mg/d in the second week; 1500 mg/d in the third week; final dose: 2000 mg/d | CNIs dose was reduced by 25% of the initial dose every week in monotherapy group | |
| Schmeding et al[28] | 2011 | Germany | Mycophenolate Mofetil Monotherapy | Mycophenolate Mofetil plus CNIs + steroids | 142 | 500 mg/d at 1 wk; 1000-1500 mg/d at 2-3 wk; 2000 mg/d thereafter | CNI dose was withdraw completely in 12 wk later | |
Tacrolimus, cyclosporine and mycophenolate mofetil were included in this meta-analysis. The initial dose of tacrolimus was between 0.05 mg/kg per day and 0.20 mg/kg per day, and subsequently adjusted to achieve target whole-blood trough levels of 10-20 ng/mL, and 5-15 ng/mL thereafter. In all related studies, cyclosporine was initially given intravenously until the patients were able to eat, and then orally at a dosage that was adjusted to maintain therapeutic levels (RiaMonokit-Whole Blood Trough Levels) between 200 and 300 ng/mL for the first month, and 150-250 ng/mL thereafter. Mycophenolate mofetil was introduced in stages: 250 mg twice daily in the first week; 500 mg twice daily in the 2nd week; 750 mg twice daily in the 3rd week; and a final dose of 1000 mg twice daily from the fourth week onwards. Anti-thymocyte globulins (ATG) were used with immunosuppression monotherapy in 5 RCTs[18,19,24-26].
The randomized studies (full-text abstracts) adhered to the majority of the guidelines, and had a mean score of 3.7 (range: 3-6) on the quality scales. Twelve RCTs[12-14,18,19,21-27] included details of drug dose and target whole-blood trough levels. The remaining studies did not explicitly describe methods of drug selection or the usage (n = 2)[20,28]. The methodological quality of the RCTs was assessed using a validated tool as described above (Table 2).
| Ref. | Selection criteria specified | Study design | Jadad scale score | Other causing of death report | Dropouts explained | Funding |
| Benítez et al[18] | Adequate | RCT | 4 | Adequate | Yes | Supported by grants from Fresenius Biotech GmbH, Spain |
| Boillot et al[19] | Adequate | RCT | 4 | Adequate | Adequate | Supported by Fujisawa GmbH, Munich, Germany |
| Weiler et al[20] | Adequate | RCT | 3 | Adequate | Adequate | NS |
| Chau et al[21] | Adequate | RCT | 3 | NS | NS | NS |
| Moench et al[22] | Adequate | RCT | 6 | Adequate | Adequate | Supported by Astellas Pharma Munich, Germany |
| Eason et al[23] | Adequate | RCT | 4 | NS | Adequate | Supported by unrestricted educational grants from Sangstat and Fujisawa |
| Samonakis et al[12] | Adequate | RCT | 3 | NS | Ns | Support from Fujisawa for some of the personnel involved in this investigator-led study |
| Margarit et al[13] | Adequate | RCT | 4 | Adequate | Adequate | Supported, in part, by a grant from Fujisawa GM |
| Manousou et al[14] | Adequate | RCT | 6 | Adequate | Adequate | NS |
| Belli et al[24] | Adequate | RCT | 3 | Adequate | Adequate | NS |
| De Carlis et al[25] | Adequate | RCT | 4 | Adequate | Adequate | NS |
| Romani et al[26] | Adequate | RCT | 4 | Adequate | Adequate | NS |
| Schlitt et al[27] | Adequate | RCT | 4 | Adequate | Adequate | NS |
| Schmeding et al[28] | Adequate | RCT | 4 | Adequate | Adequate | Funding for patient monitoring and laboratory diagnostics and data analysis was partially provided by Roche Pharmaceutical Company, Grenzach-Wyhlen, Germany |
Those studies without detailed information on drug doses or which used various doses of immunosuppression were deemed to have at least a moderate risk of bias. And in few monotherapy groups, using of ATG was deemed to have at least a moderate risk of bias too.
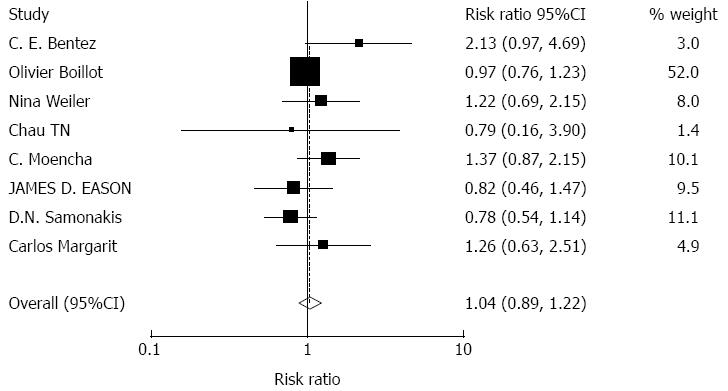
Effects of tacrolimus monotherapy on graft rejection: A total of 1214 patients were included in 8 articles about graft rejection. There were no significant effects on acute rejection with tacrolimus monotherapy (RR = 1.04, 95%CI: 0.89-1.22, Z = 0.48, P = 0.629). Tacrolimus monotherapy did not increase the risk of acute rejection, Figure 2.
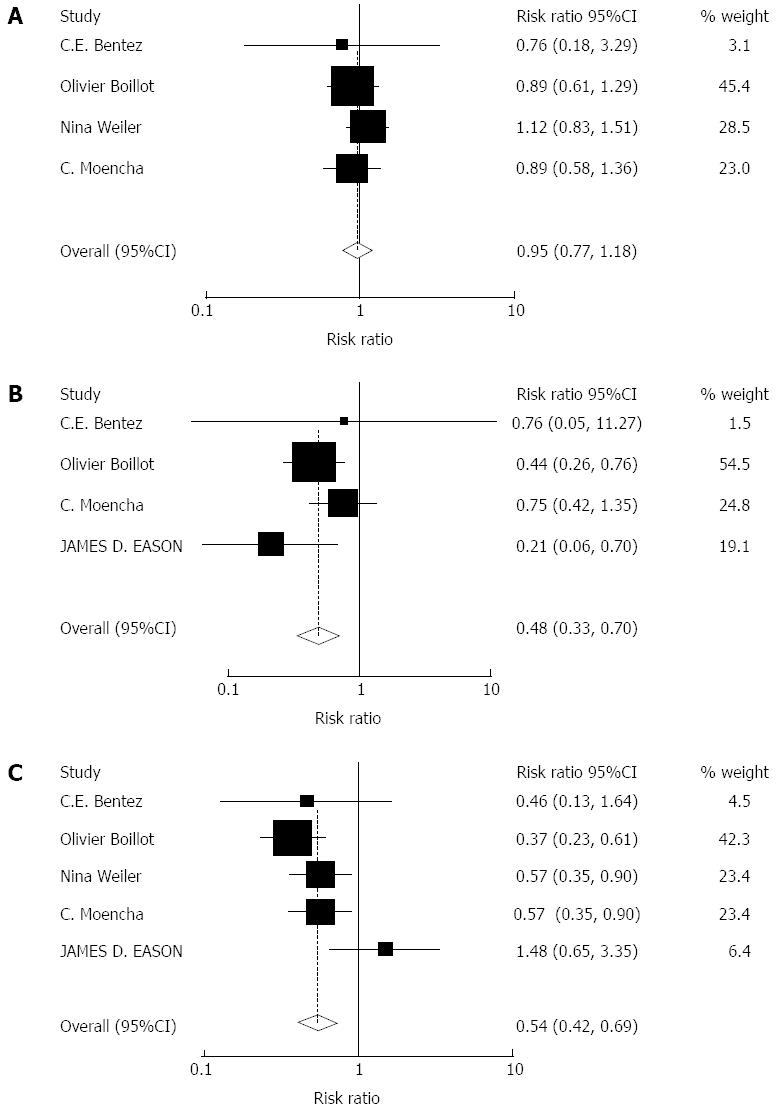
Impact of tacrolimus monotherapy on adverse events: A total of 964 patients were included in 4 articles on cytomegalovirus (CMV) infection. A total of 1084 patients were included in 5 articles on drug-related diabetes mellitus (DM), and a total of 965 patients were included in 4 articles on drug-related hypertension (HP). There were no significant effects on the drug-related HP with tacrolimus monotherapy (RR = 0.95, 95%CI: 0.77-1.18, Z = 0.46, P = 0.646). There were significant effects on the CMV infection (RR = 0.48, 95%CI: 0.33-0.70, Z = 3.86, P = 0.000) and drug-related DM (RR = 0.54, 95%CI: 0.42-0.69, Z = 4.75, P = 0.000) with tacrolimus monotherapy. More adverse events were observed in the immunosuppression combination therapy groups, Figure 3.
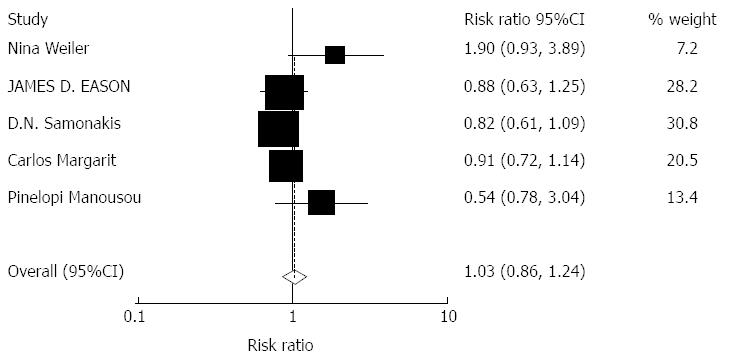
Impact of tacrolimus monotherapy on HCV recurrence: Based on our selection criteria, the effect of tacrolimus monotherapy on HCV recurrence rates was reported in 5 studies including 285 patients. The overall pooled incidence of HCV recurrence was 1.03 (95%CI: 0.86-1.24, Z = 0.32, P = 0.752). No significant effects were observed, Figure 4.
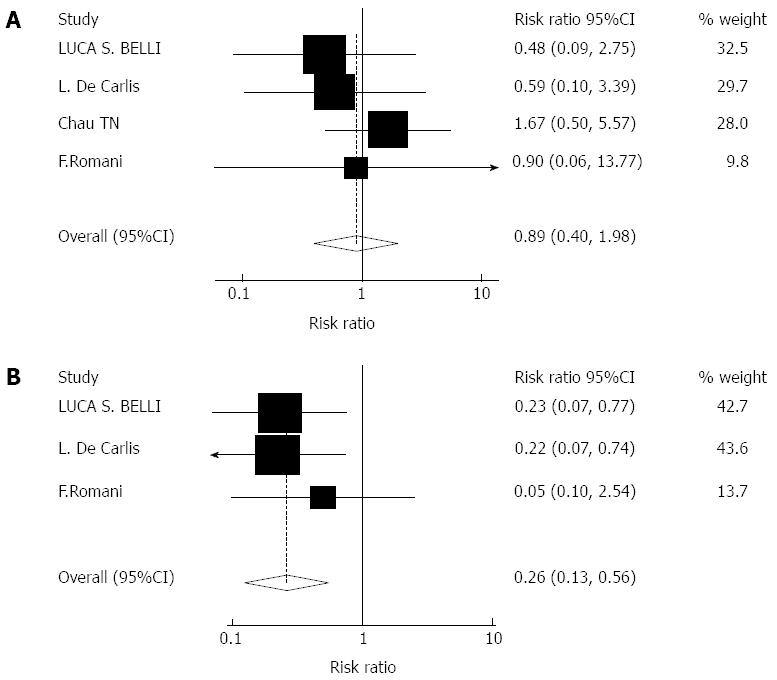
Impact of cyclosporine monotherapy on graft rejection and adverse events: Four studies including 273 patients reported the impact of cyclosporine monotherapy on graft rejection. Three studies including 262 patients reported the postoperative incidence of complications such as drug-related DM. There were no significant effects on acute rejection rates with cyclosporine monotherapy (RR = 0.89, 95%CI: 0.40-1.98, Z = 0.29, P = 0.771). There were significant effects on drug-related DM (RR = 0.26, 95%CI: 0.13-0.56, Z = 3.50, P = 0.000) with cyclosporine monotherapy, Figure 5A and B.
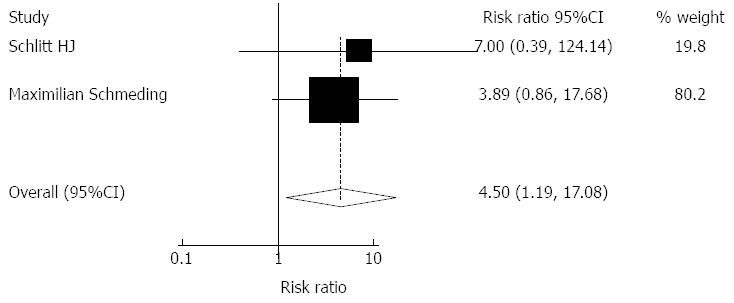
Impact of MMF monotherapy on graft rejection: Based on our selection criteria, the impact of MMF monotherapy on graft rejection was reported in 2 studies including 170 patients. The overall pooled incidence of acute rejection was 4.54 (95%CI: 1.19-17.10, Z = 2.21, P = 0.027). MMF monotherapy led to significantly more acute rejection events. Heterogeneity was tested and was found to be not statistically significant (χ2 = 0.13, P = 0.77) (Figure 6).
Meta-regression assessment for heterogeneity: To assess heterogeneity in the test performance, meta-regression assessment was performed in those studies in which P value of statistical heterogeneity were below 0.1.
P value of statistical heterogeneity below 0.1 was observed in 2 meta-analyses (Figures 3C and 4). The results of meta-regression showed that the publication year (Z = -0.17, P = 0.87) and immunosuppression dosage (Z = 0.77, P = 0.44) had no effect on heterogeneity about tacrolimus monotherapy on drug-related DM. The publication year (Z = 1.90, P = 0.05) and immunosuppression dosage (Z = 1.99, P = 0.04) may have a significant effect on heterogeneity about tacrolimus monotherapy on HCV recurrence.
The current study, derived from 14 randomized studies, is to our knowledge, the first meta-analysis that has evaluated data from multiple studies to assess the effect on graft rejection of an immunosuppression with or without corticosteroids. This meta-analysis shows that the tacrolimus and cyclosporine monotherapy may be as effective as immunosuppression by steroid-based combination therapy for liver transplantation, and is associated with fewer complications. Mycophenolate mofetil monotherapy is not recommended post-transplantation because of a high rate of acute rejection events. Tacrolimus monotherapy did not increase HCV recurrence in HCV-infected liver transplant recipients.
Recent refinements in immunosuppression therapy have led to a progressively increased survival rate after liver transplantation during the last decade[29,30]. Nonetheless, there is still a need to define the most effective immunosuppression with the minimum long-term side effects in order to improve the quality of life in solid organ transplant recipients. In particular, steroids cause undesirable metabolic effects such as post-transplant diabetes mellitus and hypertension leading to increased infection rates, osteoporosis, and potentially HCV recurrence[31,32]. The argument for complete withdrawal of steroids from chronic immunosuppression protocols gained force in the early nineties[33,34], although it was previously proposed for children in the late 1980s[7]. It seems logical to try to eliminate steroids from chronic immunosuppression, considering the following facts: steroid therapy has a non-specific and largely unelucidated immunosuppression effect (empirical use); steroid dosage can be reduced. In addition, reducing immunosuppression combination therapy after liver transplantation is justifiable to avoid various side effects of immunosuppression drugs[35,36]. A number of studies have been performed to identify an immunosuppression regimen with minimal adverse effects and maximum safety[37-39]. However, steroid-based immunosuppression combination therapy is routinely and generally used for liver transplantation.
Although steroid adjuvant and immunosupressive combination therapy are still the most popular strategies because of fewer acute rejection events, the current meta-analysis indicates that calcineurin-inhibitors alone are effective, and have fewer adverse events after liver transplantation.
The strengths of our review include a focus on immunosupression strategy, the search strategy, duplicate study elimination, and data extraction, a comprehensive search methodology, inclusion of randomized studies, the reporting of inclusion and exclusion criteria, and detailed assessment of the factors that influence the confidence in the results. In addition, we excluded case-control and cohort studies to guarantee the quality of included articles. The study provides data on various immunosupressives (tacrolimus, cyclosporine and MMF), meta-regression and drug-related adverse events that were not available from previous studies.
There are limitations of the study. The lack of standardization of immunosupression dosage is one of them. Even the meta-regression here suggested that the dosage had significant effects on heterogeneity about tacrolimus monotherapy on drug-related DM. The oral dosage was not completely consistent in all 14 articles. Dosages can vary in many ways, including drug discontinuation because of severe complications, changes in the immunosupression scheme because of acute rejection, and the compliance of patients. The drug whole-blood trough levels resulting from various dosages have effects on immunological rejection. Only a few studies explored dosage differences. Another limitation of this study was the varying times for steroids withdrawal after liver transplantation. The inclusion of various immunosuppression combinations increased the risk for bias. For example, studies included tacrolimus monotherapy vs tacrolimus, cyclosporine, and azathioprine triple therapy instead of tacrolimus monotherapy vs tacrolimus with steroid in one study[21]. In 5 articles, the immunosuppression scheme of monotherapy group was tacrolimus or cyclosporine with ATG or other globulin[18,19,24-26]. Finally, there was publication bias as well, and some of the randomized studies included in our analysis were not well designed: small sample size, patient withdrawals and switching between control and monotherapy group because of adverse events. The scarcity of such trials necessitated the inclusion of other types of studies in our analysis. There were a limited number of studies with long-term follow-up. We cannot deny the possibility of bias introduced by the tendency of subjects to dropout of studies employing lengthy follow-up periods. It is possible that unsuccessful cases were less likely to remain in these studies.
There are several reports about the relationship between recurrences and immunosuppression for liver transplantation due to HCC[40-43]. They claim that the negative impact of immunosuppression is limited. However, other reports suggest that intense treatment is a major risk factor for cancer recurrence, especially among renal transplant patients[44,45]. Medical management of transplant patients has consistently changed in the past few years because of increased clinical experience. In particular, one of the possible key factors in controlling the response to neoplasms, pharmacologic immunosuppression, is now handled with more confidence, aiming to reduce the administration schedule to the minimum in the shortest time possible.
In conclusion, the current meta-analysis found that tacrolimus and cyclosporine monotherapy may be as effective as the immunosuppression combination therapy after transplantation and effectively reduce immunosuppression-related complications. However, mycophenolate mofetil monotherapy results failed to show an association between immusupression monotherapy and the graft survival rate, the patient long term survival rate. Some of analysis on adverse events included only a few studies (less than 5). There were only two randomized studies on MMF monotherapy and graft rejection. More multi-center randomized controlled studies will be needed. It is critical to study the relationship between immusupression monotherapy and patient economic benefits. This review highlights the need for more data from longitudinal studies involving measurements of patient costs for immusupression therapies. Further studies are required to improve our understanding of the underlying mechanisms linking immune response to immunosuppression.
Immunosuppression is used to reduce rejection after liver transplantation. There is considerable controversy regarding whether an immunosuppression monotherapy should be used after transplantation. To assess the advantages (lower adverse events such as hypertension) and disadvantages (more episodes of rejection) of immunosuppression monotherapy after transplantation. To assess the impact of immunosuppression monotherapy on recurrence of hepatitis C.
Immunosuppression is an important drag protocol after organ transplantation to prevent rejection. In the area of the immunosuppression strategy, the research hotspot is how to mollify the side effect of immunosuppression and the post transplantation supporting cost. And even recent refinements in immunosuppression therapy have led to a progressively increased survival rate after liver transplantation during the last decade. So immunosuppression monotherapy may be a considerable strategy.
Although many multiple-center or single-center randomize studies have been done, this is the first meta-analysis that has evaluated data from multiple studies to assess the effect on graft rejection of an immunosuppression with or without corticosteroids. Henceforth, the authors have conclusions of this issue at the level of evidence-based medicine.
To provide a new immunosuppression protocol or strategy; to reduce the complication that suffered from immunosuppression combination and the cost post transplantation.
This review manuscript focused on the efficacy of an immunosuppression monotherapy to prevent rejection after liver transplantation, which is solid and with clear conclusions.
P- Reviewer: Lorenzo-Zuniga V, Saeed T, Zhou PH S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Bonaccorsi-Riani E, Sempoux C, Piette N, Julliard O, Kabamba B, Ciccarelli O, Roggen F, De Reyck C, Hassoun Z, Lerut J. Impact of steroid-avoidance immunosuppression on long-term outcome after liver transplantation for HCV cirrhosis: the need for well documented long-term follow-up. Acta Gastroenterol Belg. 2012;75:411-418. [PubMed] |
| 2. | Stegall MD, Everson G, Schroter G, Bilir B, Karrer F, Kam I. Metabolic complications after liver transplantation. Diabetes, hypercholesterolemia, hypertension, and obesity. Transplantation. 1995;60:1057-1060. [PubMed] |
| 3. | Jindal RM, Sidner RA, Hughes D, Pescovitz MD, Leapman SB, Milgrom ML, Lumeng L, Filo RS. Metabolic problems in recipients of liver transplants. Clin Transplant. 1996;10:213-217. [PubMed] |
| 4. | Marubashi S, Umeshita K, Asahara T, Fujiwara K, Haga H, Hashimoto T, Hatakeyama K, Ichida T, Kanematsu T, Kitajima M. Steroid-free living donor liver transplantation for HCV--a multicenter prospective cohort study in Japan. Clin Transplant. 2012;26:857-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Goddard S, Adams DH. Methylprednisolone therapy for acute rejection: too much of a good thing? Liver Transpl. 2002;8:535-536. [PubMed] |
| 6. | Martínez-Llordella M, Lozano JJ, Puig-Pey I, Orlando G, Tisone G, Lerut J, Benítez C, Pons JA, Parrilla P, Ramírez P. Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest. 2008;118:2845-2857. [PubMed] |
| 7. | Margarit C, Martínez Ibañez V, Tormo R, Infante D, Iglesias H. Maintenance immunosuppression without steroids in pediatric liver transplantation. Transplant Proc. 1989;21:2230-2231. [PubMed] |
| 8. | Padbury RT, Gunson BK, Dousset B, Hubscher SG, Buckels JA, Neuberger JM, Elias E, McMaster P. Steroid withdrawal from long-term immunosuppression in liver allograft recipients. Transplantation. 1993;55:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 95] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Dunn SP, Falkenstein K, Lawrence JP, Meyers R, Vinocur CD, Billmire DF, Weintraub WH. Monotherapy with cyclosporine for chronic immunosuppression in pediatric liver transplant recipients. Transplantation. 1994;57:544-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Fraser GM, Grammoustianos K, Reddy J, Rolles K, Davidson B, Burroughs AK. Long-term immunosuppression without corticosteroids after orthotopic liver transplantation: a positive therapeutic aim. Liver Transpl Surg. 1996;2:411-417. [PubMed] |
| 11. | Stegall MD, Everson GT, Schroter G, Karrer F, Bilir B, Sternberg T, Shrestha R, Wachs M, Kam I. Prednisone withdrawal late after adult liver transplantation reduces diabetes, hypertension, and hypercholesterolemia without causing graft loss. Hepatology. 1997;25:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Samonakis DN, Mela M, Quaglia A, Triantos CK, Thalheimer U, Leandro G, Pesci A, Raimondo ML, Dhillon AP, Rolles K. Rejection rates in a randomised trial of tacrolimus monotherapy versus triple therapy in liver transplant recipients with hepatitis C virus cirrhosis. Transpl Infect Dis. 2006;8:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Margarit C, Bilbao I, Castells L, Lopez I, Pou L, Allende E, Escartin A. A prospective randomized trial comparing tacrolimus and steroids with tacrolimus monotherapy in liver transplantation: the impact on recurrence of hepatitis C. Transpl Int. 2005;18:1336-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Manousou P, Samonakis D, Cholongitas E, Patch D, O’Beirne J, Dhillon AP, Rolles K, McCormick A, Hayes P, Burroughs AK. Outcome of recurrent hepatitis C virus after liver transplantation in a randomized trial of tacrolimus monotherapy versus triple therapy. Liver Transpl. 2009;15:1783-1791. [PubMed] |
| 15. | Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration. 2009; Available from: http://www.cochrane-handbook.org. |
| 16. | Gluud C, Nikolova D, Klingenberg SL, Whitfield K, Alexakis N, Als-Nielsen B. Cochrane Hepato-biliary Group: About The Cochrane Collaboration (Cochrane Review Groups [CRGs]). The Cochrane Library, 2008 (1): LIVER. Available from: http://onlinelibrary.wiley.com/cochranelibrary/search. |
| 17. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [PubMed] |
| 18. | Benítez CE, Puig-Pey I, López M, Martínez-Llordella M, Lozano JJ, Bohne F, Londoño MC, García-Valdecasas JC, Bruguera M, Navasa M. ATG-Fresenius treatment and low-dose tacrolimus: results of a randomized controlled trial in liver transplantation. Am J Transplant. 2010;10:2296-2304. [PubMed] |
| 19. | Boillot O, Mayer DA, Boudjema K, Salizzoni M, Gridelli B, Filipponi F, Trunecka P, Krawczyk M, Clavien PA, Ducerf C. Corticosteroid-free immunosuppression with tacrolimus following induction with daclizumab: a large randomized clinical study. Liver Transpl. 2005;11:61-67. [PubMed] |
| 20. | Weiler N, Thrun I, Hoppe-Lotichius M, Zimmermann T, Kraemer I, Otto G. Early steroid-free immunosuppression with FK506 after liver transplantation: long-term results of a prospectively randomized double-blinded trial. Transplantation. 2010;90:1562-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Chau TN, Quaglia A, Rolles K, Burroughs AK, Dhillon AP. Histological patterns of rejection using oral microemulsified cyclosporine and tacrolimus (FK506) as monotherapy induction after orthotopic liver transplantation. Liver. 2001;21:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Moench C, Barreiros AP, Schuchmann M, Bittinger F, Thiesen J, Hommel G, Kraemer I, Otto G. Tacrolimus monotherapy without steroids after liver transplantation--a prospective randomized double-blinded placebo-controlled trial. Am J Transplant. 2007;7:1616-1623. [PubMed] |
| 23. | Eason JD, Nair S, Cohen AJ, Blazek JL, Loss GE. Steroid-free liver transplantation using rabbit antithymocyte globulin and early tacrolimus monotherapy. Transplantation. 2003;75:1396-1399. [PubMed] |
| 24. | Belli LS, de Carlis L, Rondinara G, Alberti AB, Bellati G, De Gasperi A, Forti D, Idèo G. Early cyclosporine monotherapy in liver transplantation: a 5-year follow-up of a prospective, randomized trial. Hepatology. 1998;27:1524-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | De Carlis L, Belli LS, Rondinara GF, Alberti A, Sansalone CV, Colella G, Aseni P, Slim AO, Forti D. Early steroid withdrawal in liver transplant patients: final report of a prospective randomized trial. Transplant Proc. 1997;29:539-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Romani F, Belli LS, De Carlis L, Rondinara GF, Alberti A, Sansalone CV, Bellati G, Zavaglia C, Fesce E, Ideo G. Cyclosporin monotherapy (after 3 months) in liver transplant patients: a prospective randomized trial. Transplant Proc. 1994;26:2683-2685. [PubMed] |
| 27. | Schlitt HJ, Barkmann A, Böker KH, Schmidt HH, Emmanouilidis N, Rosenau J, Bahr MJ, Tusch G, Manns MP, Nashan B. Replacement of calcineurin inhibitors with mycophenolate mofetil in liver-transplant patients with renal dysfunction: a randomised controlled study. Lancet. 2001;357:587-591. [PubMed] |
| 28. | Schmeding M, Kiessling A, Neuhaus R, Heidenhain C, Bahra M, Neuhaus P, Neumann UP. Mycophenolate mofetil monotherapy in liver transplantation: 5-year follow-up of a prospective randomized trial. Transplantation. 2011;92:923-929. [PubMed] |
| 29. | Castroagudín JF, Molina-Pérez E, Ferreiro-Iglesias R, Varo-Pérez E. Strategies of immunosuppression for liver transplant recipients with hepatocellular carcinoma. Transplant Proc. 2011;43:711-713. [PubMed] |
| 30. | Manzia TM, Sforza D, Angelico R, Bellini MI, Ciano P, Manuelli M, Toti L, Tisone G. Everolimus and enteric-coated mycophenolate sodium ab initio after liver transplantation: midterm results. Transplant Proc. 2012;44:1942-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | van Hoek B, de Rooij BJ, Verspaget HW. Risk factors for infection after liver transplantation. Best Pract Res Clin Gastroenterol. 2012;26:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Tinti F, Mitterhofer AP, Muiesan P. Liver transplantation: role of immunosuppression, renal dysfunction and cardiovascular risk factors. Minerva Chir. 2012;67:1-13. [PubMed] |
| 33. | Andrews WS, Shimaoka S, Sommerauer J, Moore P, Hudgins P. Steroid withdrawal after pediatric liver transplantation. Transplant Proc. 1994;26:159-160. [PubMed] |
| 34. | Ghio L, Tarantino A, Edefonti A, Mocciaro A, Giani M, Guerra L, Berardinelli L, Vegeto A. Advantages of cyclosporine as sole immunosuppressive agent in children with transplanted kidneys. Transplantation. 1992;54:834-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Li H, Zhang J, He JW, Wang K, Wang GS, Jiang N, Fu BS, Wang GY, Yang Y, Chen GH. Symptomatic osteonecrosis of the femoral head after adult orthotopic liver transplantation. Chin Med J (Engl). 2012;125:2422-2426. [PubMed] |
| 36. | Charco R, Bilbao I, Chavez R, Castells LI, Hidalgo E, Margarit C. Low incidence of hypercholesterolemia among liver transplant patients under tacrolimus monotherapy immunosuppression. Transplant Proc. 2002;34:1555-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 37. | Reding R, Gras J, Bourdeaux C, Wieers G, Truong QD, Latinne D, Sokal E, Janssen M, Lerut J, Otte JB. Stepwise minimization of the immunosuppressive therapy in pediatric liver transplantation. A conceptual approach towards operational tolerance. Acta Gastroenterol Belg. 2005;68:320-322. [PubMed] |
| 38. | Mukherjee S, Botha JF, Mukherjee U. Immunosuppression in liver transplantation. Curr Drug Targets. 2009;10:557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Lee JH, Lee SK, Lee HJ, Seo JM, Joh JW, Kim SJ, Kwon CH, Choe YH. Withdrawal of immunosuppression in pediatric liver transplant recipients in Korea. Yonsei Med J. 2009;50:784-788. [PubMed] |
| 40. | Vivarelli M, Bellusci R, Cucchetti A, Cavrini G, De Ruvo N, Aden AA, La Barba G, Brillanti S, Cavallari A. Low recurrence rate of hepatocellular carcinoma after liver transplantation: better patient selection or lower immunosuppression? Transplantation. 2002;74:1746-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Zieniewicz K, Patkowski W, Nyckowski P, Alsharabi A, Michałowicz B, Pawlak J, Paluszkiewicz R, Wróblewski T, Najnigier B, Smoter P. Results of liver transplantation for hepatocellular cancer. Ann Transplant. 2007;12:11-14. [PubMed] |
| 42. | Valdivieso A, Bustamante J, Gastaca M, Uriarte JG, Ventoso A, Ruiz P, Fernandez JR, Pijoan I, Testillano M, Suarez MJ. Management of hepatocellular carcinoma recurrence after liver transplantation. Transplant Proc. 2010;42:660-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Matsuno N, Iwamoto H, Nakamura Y, Hama K, Kihara Y, Konno O, Jojima Y, Akashi I, Mijiti A, Ashizawa T. ABO-incompatible adult living donor liver transplantation for hepatocellular carcinoma. Transplant Proc. 2008;40:2497-2500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Watorek E, Boratynska M, Smolska D, Patrzalek D, Klinger M. Malignancy after renal transplantation in the new era of immunosuppression. Ann Transplant. 2011;16:14-18. [PubMed] |
| 45. | Berardinelli L, Raiteri M, Ghio L, Messa PG, Montagnino G. The role of immunosuppression in malignancies among 351 pediatric renal transplant patients. Transplant Proc. 2010;42:1166-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |