Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12301
Revised: May 15, 2014
Accepted: June 12, 2014
Published online: September 14, 2014
Processing time: 302 Days and 18 Hours
AIM: To test efficacy and durability of a polyphenol-based prebiotic treatment for acute gastroenteritis in a 300 patient double-blinded clinical study.
METHODS: A two-arm randomized, double-blinded, placebo-controlled clinical study was conducted at two public health centers in Managua, Nicaragua. Potential subjects who qualified based on inclusion and exclusion criteria were randomly assigned to one of two treatment arms. Two thirds of the subjects (n = 200) received a single titrated 0.5-2 ounce liquid dose of a novel polyphenol-based prebiotic (AlivaTM) diluted with 2 to eight ounces of oral rehydration solution (ORS). One third of the subjects (n = 100) were randomized to receive two liquid ounces of a taste and color-matched placebo diluted in eight ounces of ORS. The outcome variables measured included stool consistency, stomach discomfort, gas and bloating, and heartburn/indigestion. The study subjects ranked their stool consistency and the severity of their subjective symptoms at specified intervals from immediately prior to treatment, to five days post treatment. All subjects recorded their symptoms in a study diary. The study subjects also recorded the time and consistencies of all stools in their study diary. Stool consistency was compared to the picture and descriptions on the Bristol Stool Chart, and any stool rated greater than Type 4 was considered unformed. The clinical study team reviewed the study diaries with subjects during daily follow-up calls and close-out visits, and recorded the data in case report forms.
RESULTS: After receiving a single dose, Aliva treated subjects reported shorter median time to their last unformed stool (1 h 50 min) than placebo treated subjects (67 h 50 min.), a statistically significant difference [95%CI: -3178-(-2018), P = 0.000]. Aliva treated subjects also reported shorter median their time to last unformed stool (TTLUS) (1hrs 50 min) than placebo treated subjects (67 h 50 min), which was also a statistically significant difference (P = 0.000).The percentage of subjects recording TTLUS was greater for those who received Aliva vs placebo at 30 min (P = 0.027), 2 h (P = 0.000), 24 h (P = 0.000), 48 h (P = 0.000), 72 h (P = 0.000), and 5 d (P = 0.000) post dose. There were 146 study subjects 14 years old or older, which was the criteria set for reliable self-reporting of subjective symptoms. Of those 146 subjects, 142 reported stomach pain and discomfort during screening. From 90 minutes [95%CI: -1.8-(-0.01), P = 0.048] through 5 d [95%CI: -3.4-(-1.9), P = 0.000), the subjects treated with Aliva experienced significantly less stomach pain and discomfort than those who received placebo. Of those same 146 participants, 114 subjects reported gas and bloating during screening. Similarly, subjects who received Aliva experienced significantly less gas and bloating from 2 h [95%CI: -1.7-(-0.39), P = 0.030] through 5 d (95%CI: -2.0-0.42, P = 0.005) compared with the placebo arm.
CONCLUSION: In this double-blind, randomized clinical study, subjects with acute gastroenteritis receiving Aliva prebiotic showed significant and sustained improvement of multiple symptoms vs those receiving placebo.
Core tip: The global standard of care for treating acute gastroenteritis in children is 5-10 d of oral rehydration therapy, which saves lives and may reduce the duration of the illness by 20%. In this double-blind, placebo-controlled clinical study, 60% of subjects treated with a novel polyphenol-based prebiotic experienced their last unformed stool within 2 h vs 25% of the placebo treated subjects, and 89% within 24 h vs 38% of the placebo treated group. This represents a potentially extraordinary advance in the clinical management of acute gastroenteritis. If these results can be confirmed in additional studies with different populations, this treatment should become the new global standard of care.
- Citation: Noguera T, Wotring R, Melville CR, Hargraves K, Kumm J, Morton JM. Resolution of acute gastroenteritis symptoms in children and adults treated with a novel polyphenol-based prebiotic. World J Gastroenterol 2014; 20(34): 12301-12307
- URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12301.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12301
Gastroenteritis is defined as the inflammation of the mucus membranes of the gastrointestinal tract and is characterized by diarrhea or vomiting[1]. Definitions of diarrhea include increases in volume or fluidity of stools, changes in consistency, and increased frequency of defecation[2]. The World Health Organization (WHO) defines diarrhea as the “passage of loose or watery stools at least three times in a 24 h period”, with more emphasis on the change in stool consistency rather than on frequency[3]. The major burden of diarrheal illness is currently experienced by the developing world, where children suffer from 6-7 episodes per year, compared to only 1 episode in developed countries[4]. Malnutrition, lack of education, inadequate access to safe water, as well as poor sanitation and hygiene contribute to the high incidence of diarrheal diseases in developing countries[5].
Acute gastroenteritis is a common childhood disease with 1.5 billion pediatric episodes worldwide per year[6], accounting for 16% of all deaths among children under the age of 5, with those in developing countries at particular risk[7]. In the early eighties, diarrheal diseases accounted for approximately 4.6 million deaths in children younger than 5 years[8]. In 1992, a review of studies conducted in the previous decade suggested that even without a significant change in incidence, diarrheal mortality in children under 5 years declined to approximately 3.3 million per year[9]. This reduction has been largely attributed to implementation of oral rehydration therapy (ORT) coordinated by the WHO[10]. Over the past ten years the number of deaths was further reduced to 1-1.5 million children[11].
In developed countries like the United States, where it accounts for 300 annual deaths, acute gastroenteritis is not a common cause of mortality[6]. In these countries the impact of acute gastroenteritis is often measured by the financial impact of morbidity, which results in a heavy burden on the health care system[12]. In the United States acute gastroenteritis accounts for 1.5 million visits to primary care providers and 220000 hospital admissions of children under 5[6].
Oral rehydration: Diarrheal illness is currently managed by the administration of oral or intravenous rehydration and the continuation of feeding, which for young infants includes breastfeeding[13]. Relatively recent studies indicate that co-administering zinc tablets with oral rehydration salts (ORS) may reduce stool volume by 20%, vomiting by 30%, and may shorten the duration of symptoms of a typical 5-7 d course by 20%[3]. Based on these data, the WHO has recommended routine administration of 10-20 mg/d zinc tablets over the course of 10-14 d, in the supportive management of diarrhea in young children, irrespective of etiology[3]. Full implementation of this recommendation in developing countries remains elusive due to challenging logistics as well as the inherent difficulty caregivers face when trying to administer a solution that does not taste good to children who are already feeling ill[4].
Antibiotics: Therapy with effective antimicrobial agents is required for management of enterohemorrhagic Escherichia coli (E. coli), Shigella sp., and severe cholera[14]. Treatment of infective diarrhea with antimicrobials is controversial[14] due to the emergence of antimicrobial resistance, which requires continuous monitoring of pathogen susceptibility and subsequent dissemination of this information to health care providers, which is often not available in the developing nations[15]. Also, the lack of a definitive recommendation has also made physicians reluctant to prescribe antimicrobials for the management of diarrhea in children. Further complicating these decisions, specialized testing[16] is necessary to identify certain bacterial strains, which when treated with antibiotics have the potential to provoke a serious hemolytic-uremic syndrome. As new treatment regimens emerge, it is unclear how the role of antimicrobial agents for the management of infective diarrhea will evolve[14].
Vaccines: Vaccines against diarrheagenic E. coli are in the development phase and are likely to require many more years before they become commercially available[17]. An oral rotavirus vaccine was determined to be effective and safe in controlled trials, yet was quickly withdrawn due to a higher risk of intussusception[18]. Since then, 2 new rotavirus vaccines have been widely distributed and monitored in the United States[19]. While promising, treatment regimens involving vaccines are economically and logistically challenging, particularly in less developed regions[20].
Purpose: The purpose of this clinical study is to determine if a treatment with a proprietary polyphenol-based prebiotic (Aliva™) is able to reduce the duration of diarrhea and the severity of concurrent symptoms, including abdominal discomfort, gas and bloating, and heartburn/indigestion in children and adults diagnosed with acute gastroenteritis.
Patients arriving at a network of referring community health centers in Managua, Nicaragua who presented with symptoms of acute gastroenteritis, including mild to moderate diarrhea, were informed that they might be eligible to participate in a double-blind, randomized, placebo-controlled, clinical study involving a single dose of a prebiotic. Those expressing interest were pre-screened for inclusion/exclusion criteria by staff physicians. Patients who qualified based on these criteria were then referred to the nearest of two participating research sites located at community health centers in Managua, Nicaragua. Prior to departing the referring health clinic, patients were given a stool sample kit, which they were instructed to present to the study coordinator during screening. They were also informed that those with stool samples testing positive for parasites would not be eligible for inclusion in this study.
Upon arrival at the research site, patients were administered a local IRB approved informed consent agreement by the principal investigator or study coordinator. The informed consent agreement and process, as well as all communication during the study were in the subjects’ native language. Consenting patients were examined by the principle investigator and then again evaluated for inclusion/exclusion criteria by the principal investigator or study research coordinator. Prospective subjects who were pregnant or lactating, or presented with high fever, vomiting, severe dehydration, bloody stools, underlying chronic disease, displayed signs of drug abuse, were under the age of 2, or had been experiencing symptoms for more than 48 h were excluded.
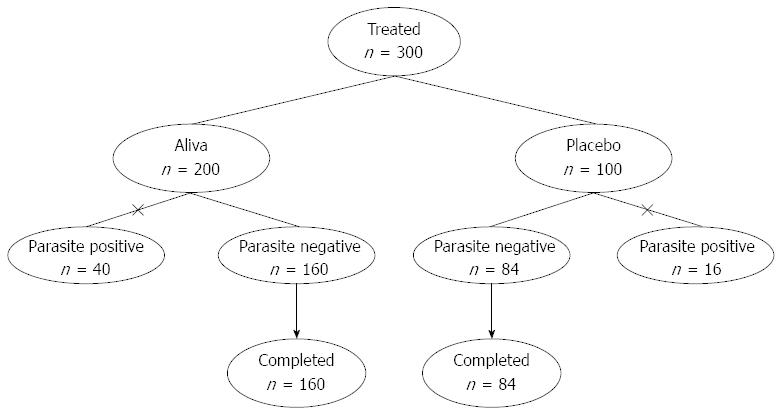
Three hundred consenting subjects qualified based on inclusion/exclusion and were assigned to receive a sequentially numbered bottle that had been randomly designated to be filled with either 2 liquid ounces of Aliva or a color, taste, and odor-matched placebo in identical packaging, on a 2:1 ratio, according to a computer-generated random number allocation code. The study was double-blinded in that neither the study site personnel (including the investigator and study coordinator) nor the subjects and their families knew of the treatment assignment, or had access to the randomization code. Two thirds of the subjects (n = 200) were administered a 2 ounce bottle filled with Aliva diluted in 8 ounces of ORS, whereas one third (n = 100) were administered a 2 ounce bottle of placebo diluted in 8 ounces of ORS.
Forty of the 200 subjects who received Aliva and 16 of the 100 subjects receiving placebo tested positive for parasites and were dropped from the current study due to failure to meet this inclusion criteria. This resulted in 244 total subjects who received Aliva (n = 160) or placebo (n = 84) (Figure 1). Among the 244 subjects evaluated, there were 133 adults and 111 children, including 43 children 2-5 years old. There were 119 males and 125 females.
Following treatment administration, subjects remained in the clinic for 2 h of observation, during which time they completed questionnaires to examine the effects of treatment on secondary outcome measures, including: stomach discomfort; gas and bloating; and heartburn/indigestion. The subjects were asked to rank the degree to which they were experiencing each of the secondary outcome measures on a scale of 0 (none) to 10 (most severe) immediately prior to, as well as 30, 60, 90 and 120 min, 24 h, and 5 d after being administered a single dose of Aliva or placebo.
Prior to clinic departure, all subjects were instructed not to take any other antidiarrheal drugs during the study period. In addition, they were not to take antacids, antiemetics, antiflatulents, antibiotics, or analgesics while participating in the study. At this time all subjects and parents of minors were provided with a 5 d supply of ORS and instructed in the completion of a diary, which is where they recorded the time and consistency of all stools for the duration of their participation in the study. The consistencies of all stools were recorded as that which was closest to the corresponding picture and description on the Bristol Stool Chart.
Subjects were contacted on days 2-4, and the information recorded in their study diaries, including adverse events, was captured on case report forms. Close-out visits were conducted in person on day 5. Statistical analysis included two-tailed comparison of categorical and continuous variables by t-test and χ2 as appropriate with P < 0.05 set as significant.
The primary outcome measure, time to last unformed stool (TTLUS) was calculated from entries in the subject diaries. TTLUS is defined as the elapsed time between initial administration of study solution and the last unformed stool, defined as any stool rated greater than 4 on the Bristol Stool Chart.
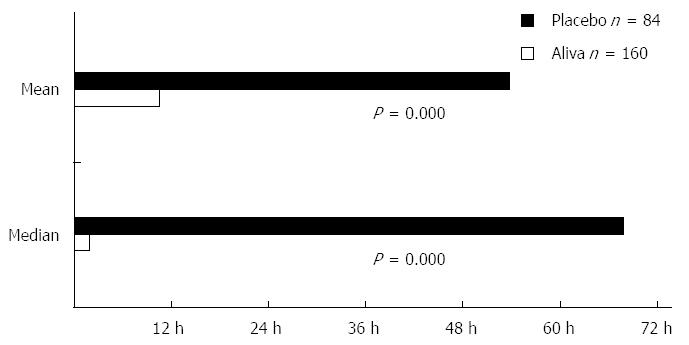
Subjects receiving Aliva experienced a shorter mean TTLUS stools (10 h 28 min) than those receiving placebo (53 h 47 min). This represented a statistically significant difference [95%CI: -3178-(-2018), P = 0.000]. Aliva treated subjects also reported shorter median TTLUS (1 h 50 min) than placebo treated subjects (67 h 50 min), which was also a statistically significant difference (P = 0.000) (Figure 2).
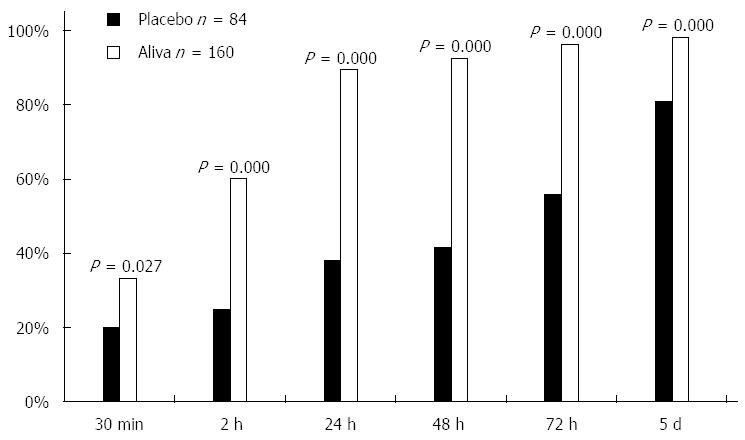
The percent of subjects recording their last unformed stool was greater for those in the Aliva treatment group than those in the placebo treatment group at 30 min (P = 0.027), 2 h (P = 0.000), 24 h (P = 0.000), 48 h (P = 0.000), 72 h (P = 0.000), and 5 d (P < 0.000) (Table 1, Figure 3).
| Aliva | Placebo | P value | |
| 30 min | 33% | 20% | 0.027 |
| 2 h | 60% | 25% | 0.000 |
| 24 h | 89% | 38% | 0.000 |
| 48 h | 93% | 42% | 0.000 |
| 72 h | 96% | 56% | 0.000 |
| 5 d | 98% | 81% | 0.000 |
Secondary outcome measures, based on self-reporting, were recorded by 146 subjects, including 133 adults and 13 children who were at least 14 years old. Children under 14 were not instructed to record secondary outcome measures because children in this age range were not expected to provide consistent and accurate self-reports.
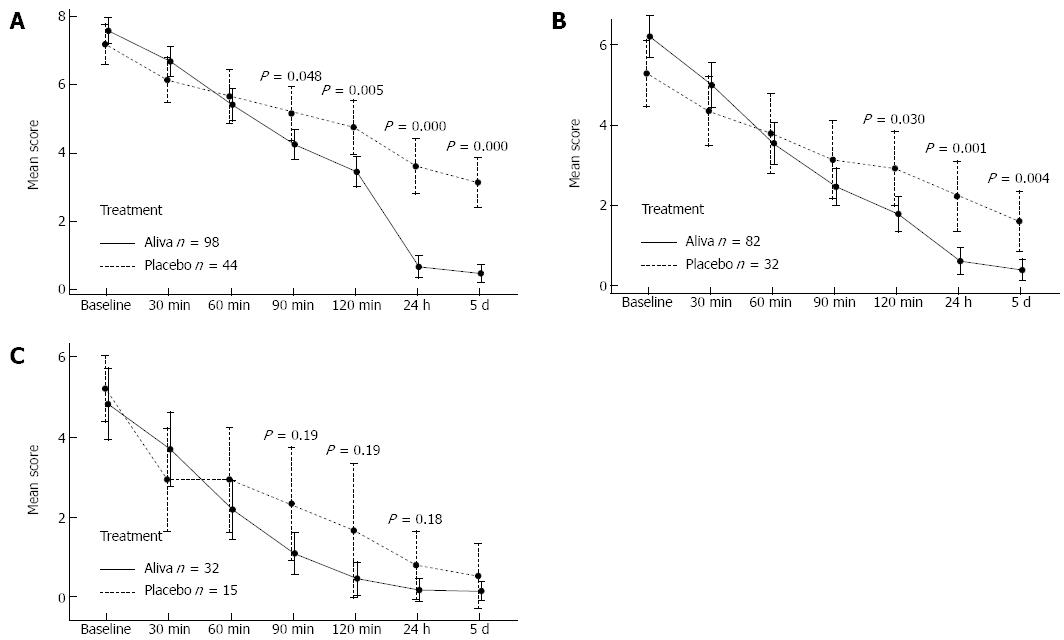
Among the 146 subjects recording secondary outcome measures, 142 (98 Aliva treatment arm and 44 placebo treatment arm) indicated that they were experiencing stomach pain and discomfort during screening. Symptoms began to improve within 30 min in subjects receiving Aliva as well as those receiving placebo, however the subjects receiving Aliva experienced significantly less stomach pain and discomfort than those receiving placebo at 90 min [95%CI: -1.8-(-0.01), P = 0.048], 2 h [95%CI: -2.2-(-0.41), P = 0.005], 24 h [95%CI: -3.8-(-2.1), P = 0.000], and 5 d [95%CI: -3.4-(-1.9), P = 0.000] (Table 2, Figure 4A).
| Aliva | Placebo | P value | 95%CI | |
| Baseline | 7.6 | 7.2 | 0.25 | |
| 30 min | 6.7 | 6.1 | 0.17 | |
| 60 min | 5.4 | 5.7 | 0.59 | |
| 90 min | 4.3 | 5.2 | 0.048 | -1.8-(-0.01) |
| 120 min | 3.4 | 4.8 | 0.005 | -2.2-(-0.41) |
| 24 h | 0.7 | 3.6 | 0 | -3.8-(-2.1) |
| 5 d | 0.5 | 3.1 | 0 | -3.4-(-1.9) |
Gas and bloating symptoms were reported by 114 subjects (82 Aliva treatment arm 1 and 32 placebo treatment arm 2 subjects) 14 and over during screening. Symptoms of gas and bloating also began to improve within 30 minutes in subjects receiving Aliva as well as those receiving placebo, however subjects who received Aliva experienced significantly less gas and bloating at 2 h [95%CI: -2.1-(-0.12), P = 0.030], 24 h [95%CI: -2.5-(-0.7), P = 0.001], and at 5 d [95%CI: -2.0-(-0.42), P = 0.004] (Table 3, Figure 4B).
| Aliva | Placebo | P value | 95%CI | |
| Baseline | 6.1 | 5.3 | 0.06 | |
| 30 min | 4.9 | 4.3 | 0.21 | |
| 60 min | 3.5 | 3.8 | 0.65 | |
| 90 min | 2.4 | 3.1 | 0.21 | -1.7-(-0.39) |
| 120 min | 1.8 | 2.9 | 0.031 | -2.1-(-0.12) |
| 24 h | 0.6 | 2.2 | 0.001 | -2.5-(-0.7) |
| 5 d | 0.4 | 1.6 | 0.004 | -2.0-(-0.42) |
Heartburn/indigestion symptoms were reported by 47 subjects (32 Aliva treatment arm 1 and 15 placebo treatment arm) 14 and over during screening. Symptoms of Heartburn/Indigestion began to improve within 30 min for subjects in subjects in both treatment arms. From 1 h (P = 0.190) through 24 h (P = 0.184) of observation, those subjects in the Aliva treatment arm experienced less heartburn/indigestion than the subjects in the placebo treatment arm, however these differences did not attain statistical significance, possibly due to the small sample sizes (Table 4, Figure 4C).
| Aliva | Placebo | P value | 95%CI | |
| Baseline | 5.0 | 5.2 | 0.51 | |
| 30 min | 3.8 | 2.9 | 0.13 | |
| 60 min | 2.3 | 2.9 | 0.63 | |
| 90 min | 1.2 | 2.3 | 0.19 | -2.2-0.46 |
| 120 min | 0.5 | 1.7 | 0.19 | -2.2-0.49 |
| 24 h | 0.2 | 0.8 | 0.18 | -1.2-0.25 |
| 5 d | 0.2 | 0.5 | 0.38 | -0.99-0.39 |
Current management of diarrheal illness in children involves prevention and management of dehydration with continued feeding and oral or intravenous rehydration as appropriate. While treatment with ORS has helped to save millions of lives per year in developing nations, approximately 1-1.5 million children under the age of five in developing nations still die from dehydration secondary to diarrhea[11]. In the United States, acute diarrheal illnesses account for 1.5 million visits to primary care providers and 10% of all hospital admissions for children under five years[6].
The results of this study indicate that Aliva significantly reduced the duration of multiple symptoms of acute gastroenteritis in children and adults presenting with acute, afebrile, mild-to-moderately severe acute gastroenteritis. For a single dose of Aliva the median time to last unformed stool during the 5 d observation period in the current study was 1 h 50 min. By contrast, in a comparable study[21] with loperamide, the median time to last unformed stool following administration of loperamide was 18 h 30 min. Highlighting the seriousness of the subjects’ condition it is also notable that in current study the median time to last unformed stool in the placebo group was 67 h 50 min, whereas it was only 27 h in the loperamide study[21].
Sixty percent of the subjects who received Aliva plus ORS experienced their last unformed stool in less than 2 h, whereas only 25% of the subjects receiving placebo plus ORS recorded their last unformed stool within 2 h (Figure 3). These results, in addition to the observed reduction in abdominal discomfort, gas and bloating, and heartburn/indigestion indicate an initial clinical response within 1-2 h. As there were no adverse events reported in this study, the results suggest that Aliva is well tolerated and effective in reducing the symptoms associated with acute gastroenteritis.
There are several limitations to this study. First, since this was a first-in-humans study our initial protocol excluded all children under 12 due to safety concerns. However, even though blinded, when physicians at the research sites started seeing dramatic recoveries without any reports of adverse events they requested a modification in the protocol that would allow for the inclusion of children. After consultation, the decision was made to allow for the inclusion of children at least two years of age. A follow-up study with children six-months and older has been planned. Second, subjects who had experienced vomiting were excluded due to inherent difficulty in determining the amount reaching the small intestine in subjects who vomited. Since vomiting is a common symptom of acute gastroenteritis and the treatment dose was well tolerated, subsequent studies will include subjects experiencing vomiting. Third, due to budget constraints, no lab tests other than stool sample screening for parasites were performed to determine etiology, which precludes determining if the effects are limited to certain viruses or bacteria. Future studies have been planned that will include additional lab testing. Fourth, while no reoccurrences of symptoms were reported during the five day follow-up period, the possibility remains that there may have been cases where symptoms recurred more than five days after treatment. Additional studies have been planned that will follow subjects for at least 10 d.
In conclusion, this study provides clinical information concerning the use of Aliva, a novel polyphenol-based prebiotic for the treatment of acute nonspecific diarrhea in children and adults. The data collected in this study demonstrate that when co-administered with oral rehydration therapy, Aliva is well tolerated and significantly shortens the duration and severity of symptoms of acute nonspecific diarrhea. A single dose of Aliva can be a useful adjunct to oral fluid therapy in a variety of circumstances and may reduce the necessary duration of oral rehydration therapy. Aliva is available in over the counter products, such as Good Gut Daily, in the United States, and is labeled for use in adults and children at least 2 years of age.
Globally, children experience 1.5 billion episodes of acute gastroenteritis each year. Oral rehydration with zinc supplements has been critical in reducing diarrhea-related mortality, and the typical 5-7 d duration diarrhea by up to 20%, however dehydration resulting from acute gastroenteritis remains a leading cause of death among children under the age of 5. While not a leading cause of death among infants and children in the developed nations, as well as in children over the age of 5 and adults everywhere, acute gastroenteritis is very common. In the US alone, children visit primary care physicians 1.5 million times annually due to acute gastroenteritis. While the use of loperamide in children is discouraged by the World Health Organization and the American Academy of Pediatrics due to concerns regarding both its safety and efficacy, it is commonly used to treat diarrhea in adults, and in a controlled clinical trial, a multi-dose regimen reduced the median time to last unformed stool by 25% vs placebo.
Current treatments for non-specific acute gastroenteritis, including oral rehydration in children and loperamide in older children and adults, may lead to marginal reduction in the duration of diarrhea. The role of polyphenol-based prebiotics has not previously been studied. This placebo-controlled study was conducted to evaluate the safety and efficacy of Aliva, a novel polyphenol-based prebiotic, for non-specific acute gastroenteritis in adults and children of at least 2 years of age. The main measured outcomes included time to last unformed stool following administration of a single titrated dose of Aliva or placebo, as well as self-reported symptoms over a 5 d period in adults and children at least 14 years of age, such as abdominal discomfort, gas & bloating, as well as heartburn/indigestion.
The authors of this study found that Aliva was well tolerated and that vs placebo, Aliva led to a rapid and sustained resolution of diarrhea and abdominal discomfort, as well as gas and bloating due to non-specific causes. A single dose of Aliva can be a useful adjunct to oral fluid therapy in a variety of circumstances and may reduce the necessary duration of oral rehydration therapy.
Subjects receiving Aliva experienced a 97% reduction in median time to last unformed stool vs placebo, 1 h 50 min vs 67 h 50 min, respectively. If proven safe and effective in additional studies, including in children less than 2 years of age, Aliva has the potential to become the new standard in treating acute gastroenteritis, and possibly other disorders related to the digestive tract.
The safety and efficacy of a novel prebiotic for treating the symptoms of acute gastroenteritis were evaluated in this well-controlled, prospective clinical study. The authors concluded that Aliva was safe and effective in reducing the symptoms of acute gastroenteritis in adults and children at least 2 years of age.
P- Reviewer: Krishnan T, Touil-Boukoffa C S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Centers for Disease Control and Prevention. Viral Gastroenteritis 2012. February 25, 2012 [cited 2013 February 3]. Available from: http://www.cdc.gov/ncidod/dvrd/revb/gastro/faq.htm. [Cited in This Article: ] |
| 2. | Mayo Foundation for Medical Education and Research. Diarrhea. 2010 June 26 [cited 2013 February 3]. Available from: http://www.mayoclinic.com/health/diarrhea/DS00292. [Cited in This Article: ] |
| 3. | World Health Organization. The treatment of diarrhoea: a manual for physicians and other senior health workers. Geneva, Switzerland; 1995. Available from: http://www.who.int/chd/publications/cdd/textrev4.htm. [Cited in This Article: ] |
| 4. | Santosham M, Keenan EM, Tulloch J, Broun D, Glass R. Oral rehydration therapy for diarrhea: an example of reverse transfer of technology. Pediatrics. 1997;100:E10. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Thapar N, Sanderson IR. Diarrhoea in children: an interface between developing and developed countries. Lancet. 2004;363:641-653. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in RCA: 226] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | King CK, Glass R, Bresee JS, Duggan C. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Elliott EJ. Acute gastroenteritis in children. BMJ. 2007;334:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Snyder JD, Merson MH. The magnitude of the global problem of acute diarrhoeal disease: a review of active surveillance data. Bull World Health Organ. 1982;60:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Bern C, Martines J, de Zoysa I, Glass RI. The magnitude of the global problem of diarrhoeal disease: a ten-year update. Bull World Health Organ. 1992;70:705-714. [PubMed] [Cited in This Article: ] |
| 10. | Victora CG, Bryce J, Fontaine O, Monasch R. Reducing deaths from diarrhoea through oral rehydration therapy. Bull World Health Organ. 2000;78:1246-1255. [PubMed] [Cited in This Article: ] |
| 11. | World Health Organization. Diarrhoeal disease. 2009 August. Available from: http://www.who.int/mediacentre/factsheets/fs330/en/index.html. [Cited in This Article: ] |
| 12. | Chow CM, Leung AK, Hon KL. Acute gastroenteritis: from guidelines to real life. Clin Exp Gastroenterol. 2010;3:97-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Wood JD. Neuropathophysiology of functional gastrointestinal disorders. World J Gastroenterol. 2007;13:1313-1332. [PubMed] [Cited in This Article: ] |
| 14. | Casburn-Jones AC, Farthing MJ. Management of infectious diarrhoea. Gut. 2004;53:296-305. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Byarugaba DK. A view on antimicrobial resistance in developing countries and responsible risk factors. Int J Antimicrob Agents. 2004;24:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in RCA: 206] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Mackell S. Traveler’s diarrhea in the pediatric population: etiology and impact. Clin Infect Dis. 2005;41 Suppl 8:S547-S552. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142-201. [PubMed] [Cited in This Article: ] |
| 18. | Bines JE. Rotavirus vaccines and intussusception risk. Curr Opin Gastroenterol. 2005;21:20-25. [PubMed] [Cited in This Article: ] |
| 19. | Centers for Disease Control and Prevention. Rotavirus. 2012 November 9, 2012. Available from: http://www.cdc.gov/vaccinesafety/vaccines/rotavsb.html. [Cited in This Article: ] |
| 20. | Babji S, Kang G. Rotavirus vaccination in developing countries. Curr Opin Virol. 2012;2:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Kaplan MA, Prior MJ, McKonly KI, DuPont HL, Temple AR, Nelson EB. A multicenter randomized controlled trial of a liquid loperamide product versus placebo in the treatment of acute diarrhea in children. Clin Pediatr (Phila). 1999;38:579-591. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |