Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9534
Revised: March 6, 2014
Accepted: April 21, 2014
Published online: July 28, 2014
AIM: To investigate the postprandial response of bone turnover markers in patients with Crohn’s disease (CD).
METHODS: Fifty nine patients with CD aged 38 ± 14 years, and 45 healthy individuals matched for age and body mass index were included in the study. All participants underwent an oral glucose tolerance test (OGTT) after an overnight fast and serum levels of the bone resorption marker C-terminal crosslinking telopeptide of type I collagen (CTX-I) and the bone formation marker procollagen type I N propeptide were measured. Activity of the disease was assessed by calculation of the Crohn’s disease activity index (CDAI).
RESULTS: Serum CTX-I was significantly higher in patients compared to controls (CTX-I: 453 ± 21 pg/mL vs 365 ± 25 pg/mL, P = 0.008), and values were significantly correlated with the activity of the disease (r = 0.435, P = 0.001). Results from OGTT-induced suppression of CTX-I showed two different trends. Patients with more active disease (assessed as CDAI > 150) had a more excessive suppression of CTX-I compared to controls (55% vs 43% P < 0.001), while patients on remission (assessed as CDAI < 150) demonstrated an attenuated CTX-I suppression (30% vs 43% P < 0.001). In line with this, CTX-I suppression after oral glucose load was significantly correlated with the activity of the disease (r = 0.913, P < 0.001).
CONCLUSION: The physiological skeletal response of postprandial suppression of bone resorption is maintained in patients with CD and is strongly dependent to the activity of the disease.
Core tip: Serum C-terminal crosslinking telopeptide of type I collagen is significantly higher in patients with Crohn’s disease (CD) compared to controls and values are significantly correlated with the activity of the disease, reflecting probably the increased osteoclastogenesis induced by the secretion of inflammatory cytokines. Despite higher bone turnover, however, the physiological skeletal response of postprandial suppression of bone resorption is maintained in patients with CD but is strongly dependent to the activity of the disease.
- Citation: Karatzoglou I, Yavropoulou MP, Pikilidou M, Germanidis G, Akriviadis E, Papazisi A, Daniilidis M, Zebekakis P, Yovos JG. Postprandial response of bone turnover markers in patients with Crohn’s disease. World J Gastroenterol 2014; 20(28): 9534-9540
- URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9534.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9534
Inflammatory bowel diseases (IBD), such as Crohn’s disease (CD) and ulcerative colitis (UC), are associated with an increased prevalence of osteoporosis, ranging from 12%-42%[1,2], with low bone mass being more common in patients with CD than UC[3,4]. Fracture risk is also increased in these patients, particularly in older individuals (> 60 years old)[5,6].
The underlying pathophysiology is still not completely understood, but the etiology seems to be multifactorial. Many of the patients with IBD receive long-term treatment with corticosteroids, and bone loss is more commonly observed in these patients[7] due to the well-established negative effects of corticosteroids on bone formation and calcium balance. Malabsorption, calcium and vitamin D deficiency, and hypogonadism due to the inhibitory effects of the disease itself as well as corticosteroid treatment effects on pituitary function are also implicated[8,9].
However, low bone mineral density (BMD) is present even in newly diagnosed patients with IBD who are not receiving corticosteroids or who have normal calcium homeostasis[10].
Recent data have illustrated the role of disease-related inflammation and secreted soluble mediators such as interleukins 1 and 6 (IL-1, IL-6), tumor necrosis factor-α (TNF-α) and receptor activator of nuclear factor kappa-B ligand (RANKL) in directly affecting bone metabolism[1,9,11,12]. In addition, treatment with the anti-TNF-α agent infliximab is associated with improvement in bone mineral density in patients with CD[1,13-15].
In the human skeleton, bones are continuously renewed, and bone resorption is equally coupled with bone formation. Several studies have shown that a variety of nutrients, including glucose[16,17] and protein[16], result in an acute suppression of bone turnover markers. In response to oral glucose in particular, there is a rapid suppression of bone resorption within minutes, with a 50% decrease in serum C-terminal crosslinking telopeptide of type I collagen (CTX-I)[18]. The greater suppression of bone resorption during oral glucose administration vs intravenous glucose administration[19] and the inhibition of this response after the administration of octreotide[17] propose a key role for gastrointestinal peptides in this physiological skeletal response.
Postprandial suppression of bone resorption has been studied in diseases that affect bone metabolism through different pathogenetic mechanisms, such as type 2 diabetes mellitus[20] and thyroid diseases[21]. The effect of inflammatory bowel diseases that disturb both bone metabolism and the secretion of gut hormones[22] is currently unknown.
In the present study, we addressed this issue by examining the response of bone turnover markers after an oral glucose load in patients with Crohn’s disease.
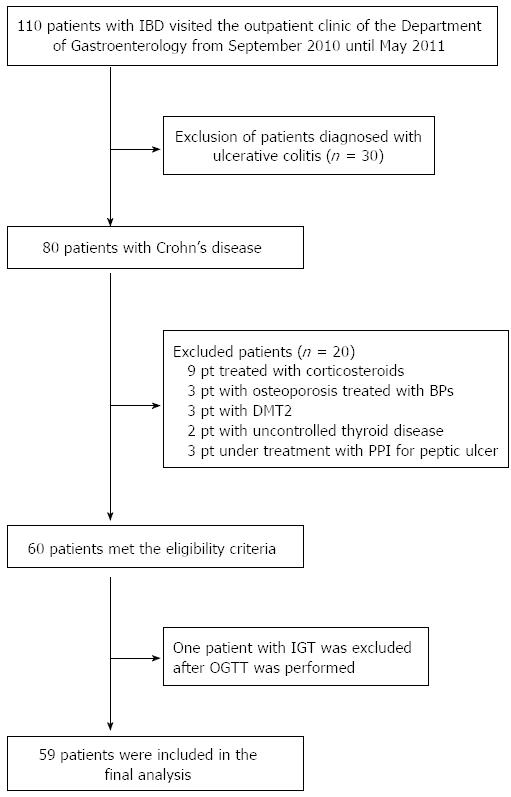
Patients with CD who were followed at the Gastroenterology Department of AHEPA University Hospital from September 2010 until May 2011 were initially screened for eligibility. Exclusion criteria were impaired renal function (serum creatinine > 120 μmol/L), impaired liver function, malignancy, thyroid disease, use of proton pump inhibitors, diabetes mellitus and the presence of diseases (i.e., primary hyperparathyroidism or Paget’s disease of bone) and/or the use of medications (i.e., glucocorticoids within 6 mo prior to enrollment, administered either locally or systematically; bisphosphonates; calcimimetics) known to affect bone metabolism. This study was approved by the local ethics committee of Aristotle University of Thessaloniki and was conducted according to the principles of the Helsinki Declaration II. Written informed consent was obtained from all participants.
The diagnosis of Crohn’s disease was based on the presence of specific endoscopic findings (aphthous ulcers, cobblestoning, areas of inflammation interspersed between normal bowel areas) or evidence from imaging studies in patients with compatible clinical symptoms (such as abdominal pain and liquid or soft stools). Disease activity was based on the Crohn’s disease activity index (CDAI) calculation, which is considered the gold standard for assessing disease activity (Table 1).
| Clinical or laboratory variable | Weighting factor |
| Number of liquid or soft stools each day for seven days | × 2 |
| Abdominal pain (graded from 0-3 on severity) each day for seven days | × 5 |
| General well being, subjectively assessed from 0 (well) to 4 (terrible) each day for seven days | × 7 |
| Presence of complications1 | × 20 |
| Taking diphenoxylate and atropine (lomotil) or opiates for diarrhea | × 30 |
| Presence of an abdominal mass (0 as none, 2 as questionable, 5 as definite) | × 10 |
| Hematocrit of < 0.47 in men and < 0.42 in women | × 6 |
| Percentage deviation from standard weight | × 1 |
One hundred and ten patients were initially screened for the study, and 59 patients were finally enrolled (Figure 1). None of the patients was supplemented with calcium and/or vitamin D at the time of the study. Forty-five healthy individuals matched for age and body mass index (BMI) who were recruited from the medical and nursing staff of the hospital were used as controls.
Of the 59 patients, 20 patients had ileal disease, 7 had colonic disease, and 32 had ileocolonic disease. Twenty-nine patients were receiving treatment with the anti-TNF-α agent, infliximab. The rest of the patients (n = 30) were mostly treated with azathioprine (AZA), while those with colonic disease (n = 7) were treated with mesalazine and/or AZA. Remission was defined as CDAI < 150 and active disease as CDAI > 150. Based on these criteria, 23 patients had active disease (39%), and 36 patients were in remission, of whom 26 (70%) were being treated with infliximab.
After an overnight fast, all participants underwent a 75 g oral glucose tolerance test (OGTT) between 0730 and 0930 h. All samples were centrifuged at 4 °C, and the serum was separated and stored at -70 °C until analysis. Serum levels of the bone formation marker procollagen type I N propeptide (P1NP) and the bone resorption marker CTX-I were assayed. For all participants, baseline levels of serum thyrotropin, serum total calcium, phosphate, PTH, 25-OH vitamin D, and creatinine were measured using standard laboratory methods. Plasma glucose levels at 0, 60, and 120 min were measured by a glucose oxidase colorimetric technique on an automated analyzer (Targa; Menarini, Florence, Italy). Data on BMD measurements assessed by dual X-ray energy absorptiometry (DXA) were retrieved from the hospital’s medical records. Osteoporosis or osteopenia was defined according to the criteria of the World Health Organization as a T-score below -2.5 or below -1.5, respectively. For young adults and premenopausal women, we also used the T-score-based definition, as has been recently proposed by the International Osteoporosis Foundation working group on osteoporosis pathophysiology[23].
Serum 25-hydroxyvitamin D levels (nmol/L) were measured using an RIA (RIA; DiaSorin, Sallugia, Italy) according to the manufacturer’s instructions. The minimum detectable concentration was 3.7 nmol/L, and the within-run and total run assay coefficients of variation were between 8.6%-12.5% and 1.9%-4%, respectively.
Serum parathyroid hormone (PTH) levels (pmol/L) were measured by an electrochemiluminescence immunoassay (ECLIA, Cobas; Roche, West Sussex, United Kingdom) according to the manufacturer’s instructions. The minimum detectable concentration was 0.1 pmol/L, and the within-run and total run assay coefficients of variation were between 0.6%-2.8% and 1.6%-3.4%, respectively. No cross-reactions were detected with osteocalcin, PTH fragment 1-37, PTH-related protein, bone-specific alkaline phosphatase, or beta-CrossLaps.
Serum P1NP and CTX-I were measured using the Roche Elecsys 2010 platform (Roche Diagnostics) according to the manufacturer’s instructions. The coefficients of variation were 5.1% for CTX-I and 1.9% for P1NP.
The results are presented as the mean ± SE. The postprandial response of bone turnover is expressed as the percent change from baseline at 2 h after oral glucose for CTX-I and P1NP, as previously described[20,21]. The differences between patients and controls were assessed using a two-tailed Student’s t-test or the non-parametric Mann-Whitney U test for independent samples, as appropriate. A paired sample t-test was used to assess the differences in bone markers during the performance of the OGTT within each group. Correlations between the percent change for CTX-I at 2 h after OGTT and activity of the disease were assessed by the Pearson correlation coefficient (r), and multivariate regression analysis was used for adjusting for confounding factors such as age, gender, duration of the disease, baseline levels of CTX-I, 25-OH-vitamin D and PTH levels. Multinomial regression analysis was used to describe the relationship between disease location (ileal, colonic and ileocolonic) and the numerical parameters of bone metabolism (25-OH vitamin D levels, percent change of CTX-I from baseline after glucose load, baseline CTX-I and P1NP levels). Data on BMD values were analyzed only descriptively due to the use of different DXA scans. A P value < 0.05 was considered statistically significant. Data were analyzed using SPSS 16.0 (SPSS Inc., Chicago, IL, United States).
The patient population studied consisted of 59 subjects (38 males and 21 females) with a mean age of 37.7 years (range 20 to 64 years). Demographic characteristics and laboratory values are depicted in Table 2.
| Healthy subjects | Crohn’s disease | P value | |
| Total number | 45 | 59 | - |
| Male | 29 (64) | 38 (64) | - |
| Premenopausal women | 8 (17) | 10 (17) | - |
| Age (yr) | 35.64 ± 2 | 37.7 ± 1.8 | NS |
| BMI (kg/cm2) | 27.1 ± 0.9 | 27 ± 0.28 | NS |
| Patients with active disease | - | 23 (39) | - |
| Disease duration (yr) | - | 7 ± 2.3 | - |
| TSH (mIU/L) | 1.74 ± 0.15 | 1.6 ± 0.79 | NS |
| PTH (pmol/L) | 4 ± 0.2 | 4.2 ± 0.12 | NS |
| 25-OH vitamin D (nmol/L) | 64.8 ± 3.2 | 31.7 ± 3 | < 0.001 |
| Serum calcium (mmol/L) | 2.2 ± 0.1 | 2.5 ± 0.08 | NS |
| Serum phosphate (mmol/L) | 1.1 ± 0.05 | 1.1 ± 0.16 | NS |
| Glucose (mmol/L) | 5.1 ± 0.1 | 4.5 ± 0.7 | NS |
| Serum creatinine (μmol/L) | 62 ± 1.9 | 71 ± 2.2 | NS |
Patients with Crohn’s disease had significantly lower values of 25-OH vitamin D compared to the control group (31.7 ± 3 nmol/L vs 64.8 ± 3.2 nmol/L, P < 0.001, respectively) (Table 2). However, PTH levels were not accordingly increased (mean values 4.2 ranging from 2 to 10 pmol/L), and only two patients (3%) developed secondary hyperparathyroidism (assessed by PTH levels > 6.1 pmol/L). Vitamin D levels were not associated with the activity of the disease or the intestinal area affected (ileal, colonic or ileocolonic disease). Seven patients (12%), of whom 2 were receiving infliximab, had osteopenia at the time of screening.
There was a wide range of bone turnover within the patient population, with serum P1NP ranging from 7.6 to 141.5 ng/mL (mean 50.8 ng/mL) and CTX-I ranging from 150 to 820 pg/mL (mean 453.5 pg/mL). Serum CTX-I was significantly correlated with serum P1NP levels (r = 0.271, P = 0.038).
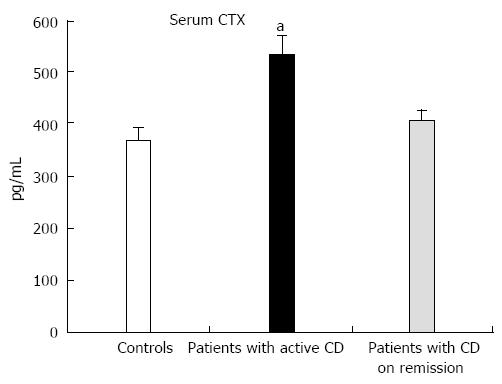
Baseline values of CTX-I but not P1NP were significantly higher in patients compared to the controls (CTX-I: 453 ± 21 pg/mL vs 365 ± 25 pg/mL, P = 0.008, and P1NP: 50.8 ± 3.7 ng/mL vs 42.4 ± 2.7 ng/mL, P = 0.074, respectively) and were significantly correlated with the activity of the disease (CDAI) (r = 0.435, P = 0.001). When patients with active disease (n = 23) and in remission (n = 36) were analyzed separately, CTX-I levels were significantly higher compared to controls only in patients with active CD (Figure 2). Vitamin D levels did not show any correlation with baseline CTX-I or P1NP levels in either group.
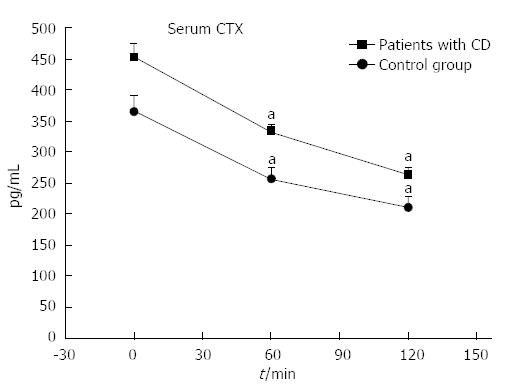
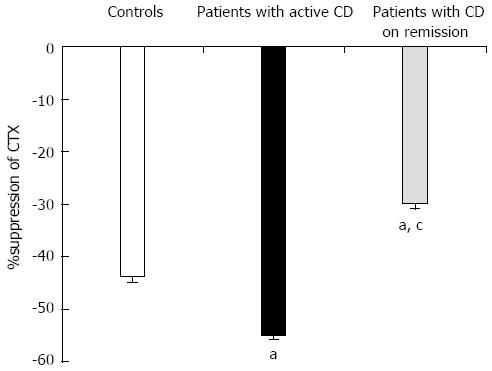
Oral glucose load significantly suppressed CTX-I levels in both patients and controls, while there was no significant change in P1NP levels (Figure 3). Patients with active disease (CDAI > 150) had more excessive suppression of CTX-I compared to controls (55% vs 43% P < 0.001), while patients in remission (CDAI < 150) demonstrated an attenuated CTX-I suppression (30% vs 43%, P < 0.001) (Figure 4). In agreement with this observation, the suppression of CTX-I after OGTT was strongly related to the activity of the disease (r = 0.913, P < 0.001), and this relationship remained robust after adjusting for age, baseline values of CTX-I, PTH or 25-OH vitamin D levels.
No significant correlations were observed between CTX-I suppression after OGTT and PTH or 25-OH vitamin D levels, duration or location of the disease.
In this study, we have shown that the physiological skeletal response of postprandial suppression of bone resorption is maintained in patients with CD but with a magnitude that differs depending on the activity of the disease.
Meal-induced suppression of bone resorption is a short-acting effect, probably mediated by non-transcriptional mechanisms and independent of gender, age and menopausal status[24].
Two recent studies have investigated the postprandial response of bone resorption in patients with type 2 diabetes mellitus[20], thyroid diseases and beta-thalassemia major[21], that are very often complicated with low bone mass and increased fracture risk. It has been shown that the postprandial suppression of bone resorption in patients with overt diabetes is significantly attenuated, suggesting an additional contributing factor in the deterioration of bone quality and bone mass observed in these patients[20]. On the other hand, in patients with hyperthyroidism and beta-thalassemia major, despite the high bone turnover state observed at baseline, the degree of postprandial suppression of bone resorption remains unaltered, indicating the significance of this response for bone homeostasis[21].
Inflammatory bowel diseases disturb bone accrual and maintenance through multiple mechanisms. Malnutrition and vitamin D deficiency are frequently encountered in this patient population, resulting in reduced calcium absorption and impaired bone mineral deposition. In our study and consistent with published data[25], 70% of the patient population had serum vitamin D levels below 50 nmol/L. However, the incidence of secondary hyperparathyroidism was not accordingly increased, suggesting that other regulators of calcium homeostasis may also be involved. Animal models of colitis proposed that vitamin D may exert an immunomodulatory role in IBD[26]. In our patients, however, we found no relationship between vitamin D levels and activity of Crohn’s disease.
Emerging evidence has proposed that intestinal inflammation plays a significant role in the development of metabolic bone disease in patients with Crohn’s disease. Increased release of several soluble cytokines, such as IL-1, IL-2, IL-6, IL-8 and TNF-α from the inflamed intestine[27-29] exert direct effects on bone, activating osteoclast precursors and promoting osteoclastogenesis[30,31].
Measurements of serum bone markers in IBD have demonstrated inconsistent results, showing either increased levels of bone resorption markers without a compensatory increase of formation markers, reduced levels of bone formation and no change in bone resorption markers[32], increased bone formation and resorption markers or no significant change between IBD patients and controls[33]. Discrepancies between studies can be attributed to small sample sizes and factors related to the heterogeneity of IBD, such as disease activity and duration, small intestinal involvement and/or resection, steroid use, age and differences in the pathophysiology of bone disease in CD vs UC[33].
In our study, patients with CD had significantly higher levels of CTX-I compared to age matched controls; these levels were significantly correlated to the activity of the disease, likely reflecting the increased osteoclastogenesis induced by the secretion of inflammatory cytokines. On the contrary, values of P1NP did not differ significantly compared to controls, but were associated with CTX-I, showing that the two processes of bone formation and resorption remain coupled.
Glucose-induced suppression of CTX-I in CD patients demonstrated two different trends. Patients who, according to the standard accepted index of activity, CDAI, were in remission demonstrated an attenuated decrease in CTX-I in comparison to the healthy controls, while patients with more active disease showed a much more excessive decrease in CTX-I. Current data cannot explain the underlying pathophysiology or the clinical relevance of this observation, and we can only speculate on the potential mechanisms involved.
It appears that more secretion of inflammatory cytokines in patients with active CD would lead to increased osteoclastogenesis and bone remodeling units compared to controls, as reflected by higher baseline CTX-I levels. In such cases, glucose load would inhibit the activity of a greater number of osteoclasts, which would be in agreement with the greater decrease in bone resorption.
However, as we have previously shown in patients with increased bone turnover due to other causes such as hyperthyroidism, suppression of bone resorption after oral glucose was of similar magnitude compared to healthy controls[23], suggesting that in CD, apart from increased bone turnover, intrinsic factors related to the disease itself may contribute to this observation.
Patients in remission demonstrated a significantly attenuated suppression of bone resorption after oral glucose load. This attenuation is probably due to the effect of anti-TNF-α treatment on osteoclasts, which inhibits osteoclastogenesis and consequently the number of osteoclasts.
Limitations of this study include the lack of data on the measurements of factors that could be involved in the mechanisms of this phenomenon, such as inflammatory cytokines or gastrointestinal peptides that are considered the key mediators of this skeletal response under physiological conditions.
Nevertheless, this is the first attempt to address the postprandial suppression of bone resorption in CD patients. Further research is needed to clarify the mechanisms that regulate this response in Crohn’s disease that severely disturbs both the functional integrity of the intestine and bone remodeling.
Inflammatory bowel diseases (IBD) disturb bone metabolism through multiple mechanisms, such as malnutrition, corticosteroid treatment and disease-related inflammation. In the normal human skeleton bone resorption is significantly reduced postprandially while it is increased during fasting, in order to provide the human body with all the necessary ingredients. Thus, the postprandial reduction in bone resorption is a physiological skeletal response that is important for bone metabolism and whole body homeostasis.
Postprandial reduction in bone resorption is attenuated significantly in female patients with type II diabetes mellitus, suggesting an additional contributing factor in the deterioration of bone quality and bone mass observed in these patients. In patients with hyperthyroidism and beta-thalassemia major, despite the high bone turnover state observed at baseline, the degree of postprandial suppression of bone resorption remains unaltered, indicating the significance of this response for bone homeostasis.
There are no similar articles, investigating the postprandial reduction of bone resorption in patients with Crohn’s disease.
This is a good study examining a poorly studied section of IBD. This report shows that glucose-induced suppression of C-terminal crosslinking telopeptide of type I collagen can be a measure of the severity of Crohn’s disease.
P- Reviewer: De Silva AP, Hokama A, Leitman M S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124:795-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 2. | van Hogezand RA, Hamdy NA. Skeletal morbidity in inflammatory bowel disease. Scand J Gastroenterol Suppl. 2006;59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Ghosh S, Cowen S, Hannan WJ, Ferguson A. Low bone mineral density in Crohn’s disease, but not in ulcerative colitis, at diagnosis. Gastroenterology. 1994;107:1031-1039. [PubMed] [Cited in This Article: ] |
| 4. | Jahnsen J, Falch JA, Aadland E, Mowinckel P. Bone mineral density is reduced in patients with Crohn’s disease but not in patients with ulcerative colitis: a population based study. Gut. 1997;40:313-319. [PubMed] [Cited in This Article: ] |
| 5. | Bernstein CN, Blanchard JF, Leslie W, Wajda A, Yu BN. The incidence of fracture among patients with inflammatory bowel disease. A population-based cohort study. Ann Intern Med. 2000;133:795-799. [PubMed] [Cited in This Article: ] |
| 6. | Vestergaard P, Krogh K, Rejnmark L, Laurberg S, Mosekilde L. Fracture risk is increased in Crohn’s disease, but not in ulcerative colitis. Gut. 2000;46:176-181. [PubMed] [Cited in This Article: ] |
| 7. | Gokhale R, Favus MJ, Karrison T, Sutton MM, Rich B, Kirschner BS. Bone mineral density assessment in children with inflammatory bowel disease. Gastroenterology. 1998;114:902-911. [PubMed] [Cited in This Article: ] |
| 8. | Compston J. Osteoporosis in inflammatory bowel disease. Gut. 2003;52:63-64. [PubMed] [Cited in This Article: ] |
| 9. | Ali T, Lam D, Bronze MS, Humphrey MB. Osteoporosis in inflammatory bowel disease. Am J Med. 2009;122:599-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | Bjarnason I, Macpherson A, Mackintosh C, Buxton-Thomas M, Forgacs I, Moniz C. Reduced bone density in patients with inflammatory bowel disease. Gut. 1997;40:228-233. [PubMed] [Cited in This Article: ] |
| 11. | Bernstein CN, Sargent M, Leslie WD. Serum osteoprotegerin is increased in Crohn’s disease: a population-based case control study. Inflamm Bowel Dis. 2005;11:325-330. [PubMed] [Cited in This Article: ] |
| 12. | Moschen AR, Kaser A, Enrich B, Ludwiczek O, Gabriel M, Obrist P, Wolf AM, Tilg H. The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut. 2005;54:479-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644-59, quiz 660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 437] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 14. | Hyams J, Damaraju L, Blank M, Johanns J, Guzzo C, Winter HS, Kugathasan S, Cohen S, Markowitz J, Escher JC. Induction and maintenance therapy with infliximab for children with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2012;10:391-9.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 15. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2987] [Cited by in F6Publishing: 2935] [Article Influence: 133.4] [Reference Citation Analysis (0)] |
| 16. | Bjarnason NH, Henriksen EE, Alexandersen P, Christgau S, Henriksen DB, Christiansen C. Mechanism of circadian variation in bone resorption. Bone. 2002;30:307-313. [PubMed] [Cited in This Article: ] |
| 17. | Clowes JA, Allen HC, Prentis DM, Eastell R, Blumsohn A. Octreotide abolishes the acute decrease in bone turnover in response to oral glucose. J Clin Endocrinol Metab. 2003;88:4867-4873. [PubMed] [Cited in This Article: ] |
| 18. | Henriksen DB, Alexandersen P, Bjarnason NH, Vilsbøll T, Hartmann B, Henriksen EE, Byrjalsen I, Krarup T, Holst JJ, Christiansen C. Role of gastrointestinal hormones in postprandial reduction of bone resorption. J Bone Miner Res. 2003;18:2180-2189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Clowes JA, Robinson RT, Heller SR, Eastell R, Blumsohn A. Acute changes of bone turnover and PTH induced by insulin and glucose: euglycemic and hypoglycemic hyperinsulinemic clamp studies. J Clin Endocrinol Metab. 2002;87:3324-3329. [PubMed] [Cited in This Article: ] |
| 20. | Chailurkit LO, Chanprasertyothin S, Rajatanavin R, Ongphiphadhanakul B. Reduced attenuation of bone resorption after oral glucose in type 2 diabetes. Clin Endocrinol (Oxf). 2008;68:858-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Yavropoulou MP, Tomos K, Tsekmekidou X, Anastasiou O, Zebekakis P, Karamouzis M, Gotzamani-Psarrakou A, Chassapopoulou E, Chalkia P, Yovos JG. Response of biochemical markers of bone turnover to oral glucose load in diseases that affect bone metabolism. Eur J Endocrinol. 2011;164:1035-1041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Besterman HS, Mallinson CN, Modigliani R, Christofides ND, Pera A, Ponti V, Sarson DL, Bloom SR. Gut hormones in inflammatory bowel disease. Scand J Gastroenterol. 1983;18:845-852. [PubMed] [Cited in This Article: ] |
| 23. | Ferrari S, Bianchi ML, Eisman JA, Foldes AJ, Adami S, Wahl DA, Stepan JJ, de Vernejoul MC, Kaufman JM. Osteoporosis in young adults: pathophysiology, diagnosis, and management. Osteoporos Int. 2012;23:2735-2748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Qvist P, Christgau S, Pedersen BJ, Schlemmer A, Christiansen C. Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone. 2002;31:57-61. [PubMed] [Cited in This Article: ] |
| 25. | Suibhne TN, Cox G, Healy M, O’Morain C, O’Sullivan M. Vitamin D deficiency in Crohn’s disease: prevalence, risk factors and supplement use in an outpatient setting. J Crohns Colitis. 2012;6:182-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648-2652. [PubMed] [Cited in This Article: ] |
| 27. | Bernstein CN, Leslie WD. The pathophysiology of bone disease in gastrointestinal disease. Eur J Gastroenterol Hepatol. 2003;15:857-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 28. | Neurath MF, Pettersson S. Predominant role of NF-kappa B p65 in the pathogenesis of chronic intestinal inflammation. Immunobiology. 1997;198:91-98. [PubMed] [Cited in This Article: ] |
| 29. | Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066-1071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3573] [Cited by in F6Publishing: 3522] [Article Influence: 130.4] [Reference Citation Analysis (0)] |
| 30. | Manolagas SC. The role of IL-6 type cytokines and their receptors in bone. Ann N Y Acad Sci. 1998;840:194-204. [PubMed] [Cited in This Article: ] |
| 31. | Redlich K, Hayer S, Ricci R, David JP, Tohidast-Akrad M, Kollias G, Steiner G, Smolen JS, Wagner EF, Schett G. Osteoclasts are essential for TNF-alpha-mediated joint destruction. J Clin Invest. 2002;110:1419-1427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 155] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Schoon EJ, Geerling BG, Van Dooren IM, Schurgers LJ, Vermeer C, Brummer RJ, Stockbrügger RW. Abnormal bone turnover in long-standing Crohn’s disease in remission. Aliment Pharmacol Ther. 2001;15:783-792. [PubMed] [Cited in This Article: ] |
| 33. | Ghishan FK, Kiela PR. Advances in the understanding of mineral and bone metabolism in inflammatory bowel diseases. Am J Physiol Gastrointest Liver Physiol. 2011;300:G191-G201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |