Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9321
Revised: March 4, 2014
Accepted: April 15, 2014
Published online: July 28, 2014
Helicobacter pylori (H. pylori) is one of the most common pathogenic bacterial infections and is found in the stomachs of approximately half of the world’s population. It is the primary known cause of gastritis, gastroduodenal ulcer disease and gastric cancer. However, combined drug therapy as the general treatment in the clinic, the rise of antibiotic-resistant bacteria, adverse reactions and poor patient compliance are major obstacles to the eradication of H. pylori. Oral site-specific drug delivery systems that could increase the longevity of the treatment agent at the target site might improve the therapeutic effect and avoid side effects. Gastroretentive drug delivery systems potentially prolong the gastric retention time and controlled/sustained release of a drug, thereby increasing the concentration of the drug at the application site, potentially improving its bioavailability and reducing the necessary dosage. Recommended gastroretentive drug delivery systems for enhancing local drug delivery include floating systems, bioadhesive systems and expandable systems. In this review, we summarize the important physiological parameters of the gastrointestinal tract that affect the gastric residence time. We then focus on various aspects useful in the development of gastroretentive drug delivery systems, including current trends and the progress of novel forms, especially with respect to their application for the treatment of H. pylori infections.
Core tip:Helicobacter pylori (H. pylori) is one of the most common pathogenic bacteria. It is the primary known cause of gastritis, gastroduodenal ulcer disease and gastric cancer. In addition to triple therapies, oral site-specific drug delivery systems (especially gastroretentive dosage forms) prolong the gastric retention time and can increase the concentration of the drug at the target site, thereby improving the therapeutic effect. This review focuses on gastroretentive drug delivery strategies and their application to the eradication of H. pylori.
-
Citation: Zhao S, Lv Y, Zhang JB, Wang B, Lv GJ, Ma XJ. Gastroretentive drug delivery systems for the treatment of
Helicobacter pylori . World J Gastroenterol 2014; 20(28): 9321-9329 - URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9321.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9321
Helicobacter pylori (H. pylori) is one of the most common pathogenic bacteria and is found in the stomachs of more than half of the world’s population. H. pylori infections are the primary known cause of gastritis, gastroduodenal ulcer disease and gastric cancer[1]. Even after 30 years of experience in H. pylori treatment, clinicians and researchers are still exploring the ideal regimens for clinical application[2].
Although H. pylori has been shown to be highly sensitive to a single antimicrobial agent in many antibacterial in vitro trials, in clinical the eradication rate of H. pylori is still low[3]. There are three explanations for this finding: first, many antibiotics are unstable in the low pH of gastric acid; second, the concentration of the drug in the deep gastric mucus where the bacterium lives is too low and third, the amount of time that the antibiotic resides in the stomach is too short[4]. Triple therapies consisting of the combined use of antibiotics are frequently used in the clinical treatment of H. pylori associated with gastroduodenal disease. However, the high level of antibiotic resistance by H. pylori, drug side effects and poor patient compliance are major drawbacks of multidrug therapy.
For these reasons, prolonging the gastric residence time of the drug while improving its stability in gastric acid is a logical approach to overcome these issues. Gastroretentive dosage forms are one of the oral site-specific drug delivery systems that have been proposed. Research into gastroretentive drug delivery systems has resulted in the development of several formulations such as floating systems, mucoadhesive/bioadhesive systems, expandable systems and magnetic systems, all of which could prolong gastrointestinal (GI) residence time to improve drug effectiveness against H. pylori[5-7].
H. pylori is a spiral gram-negative bacterium commonly found in the stomach. It was discovered and identified in 1982 by the Australian scientists Barry Marshall and Robin Warren. More than half of the world’s population harbors H. pylori in their GI tract, including up to 70% of people in developing countries and 25%-50% in developed countries[8]. H. pylori is well known to also cause several GI diseases, such as peptic ulcers and gastric carcinoma. In some countries, H. pylori infection has been shown to increase the risk of gastric cancer four-fold and even higher[9-12]. In gastric biopsy specimens, H. pylori bacteria are 2.5-5.0 μm long and 0.5-1.0 μm wide and have four to six unipolar sheathed flagella[13,14].
Most H. pylori localize deep in the less acidic region of the gastric mucus layer, which is more hospitable for survival, and do not directly interact with host cells[15]. H. pylori secretes urease, which hydrolyzes urea to ammonia and carbonic dioxide. These products can neutralize the acidic environment of the stomach and result in the release of toxins[16].
Although immune cells can normally recognize H. pylori and induce immune responses, H. pylori exert resistance to local immune responses by reducing the recognition of immune sensors and interfering with the uptake of antigens, as well as by other genetic mechanisms[17-19].
H. pylori is highly adapted to colonize the human stomach, whereas most other bacteria cannot persist in the low pH environment. H. pylori secretes toxins and other effector molecules[20] and stimulates numerous signaling pathways[21]. The primary pathogenic factors of H. pylori are altered local acid homeostasis, disruption of the gastric mucosal barrier, induction of gastric inflammation and resistance to the immune response[22,23]. Some studies have found that the secretion of vacuolating toxin A and γ-glutamyl transpeptidase both contribute to H. pylori persistence in the gastric niche and toimmune tolerance[24].
Recent findings observed abnormalities in the tight junction complexes in patients with H. pylori infections[25-27], which indicated that H. pylori infection can increase gastric mucosal permeability and result in disruption of the gastric mucosal barrier. Acid secretion studies demonstrated that increased acid secretion occurred upon H. pylori infection, resulting in local inflammation[28,29].
The recommended H. pylori treatment is a combination therapy with a proton pump inhibitor and two of the following antibiotics for 7, 10 or 14 d: clarithromycin, amoxicillin and metronidazole. However, in clinical practice, at least 20% of the patients might relapse[5,30,31], and in some Western countries, the cure rate is only 25%-60%[32]. Drug resistance is considered the primary reason for the observed ineffectiveness[33-35]. Currently, sequential therapy and non-bismuth quadruple therapy are the trends in the treatment of H. pylori. Several studies have shown that these novel treatment schedules are superior to triple therapy with regard to antibiotic resistance and the clinical eradication rate[36-41].
Recently, a “hybrid” therapy that combines sequential and concomitant therapies has been considered as a novel innovation[42-44]. Hsu et al[42] observed that the eradication rate using hybrid therapy was greater than 95%.
Although there is a high rate of eradication of H. pylori in clinical practice, bacterial resistance, side effects and poor patient compliance are major drawbacks of the multidrug therapies.
Based on H. pylori pathophysiology and the problems in clinical practice, oral site-specific drug delivery systems that can prolong the residence time at the reaction site are considered to be an ideal strategy. Gastroretentive dosage forms as a novel site-specific system could potentially improve the stability of antibiotics in gastric acid by employing different formulation strategies and allowing the antibiotic to localize to the target site in the stomach by increasing residence time[45].
There are several factors that affect the gastric retention time of oral drugs, including physiological problems and pharmaceutical factors.
Gastric emptying is a brief but key factor associated with the physiological problems. One of the primary functions of the stomach is to digest food, which involves the process of gastric emptying. To understand the influence of oral dosage gastric retention, the factors that control gastric emptying should be known. Table 1 shows the primary influential factors of gastric emptying.
Two important features that affect the oral dosage gastric residence time are the density and size of the drug. The density of the dosage determines the location and amount of time the drug resides in the stomach. Low-density systems can float on top of the gastric juice, where as high-density systems sink to the bottom of the stomach. Both positions could prolong the gastricretention time.
Size is important in designing an oral dosage form. The diameter of the human pyloric sphincter is 12 ± 7 mm[53]. Although there is no unified standard regarding dependence of the dosage size on gastric emptying, many scientists have studied the relationship between gastric emptying and tablet size. Khosla et al[54] and Coupe et al[55] observed that the tablets greater than 13 mm in diameter were more difficult to disintegrate and less likely to be emptied from a full stomach in humans. Recent studies showed that micro- or nano-sized delivery systems are a more efficient form for targeting agent. In addition to dosage density and size, the physiochemical properties of the active agent, e.g., the molecular weight and lipophilicity of the drug[56], are important parameters. Shah et al[4] showed that gastric mucus is more permeable to small molecules. Larhed et al[57] demonstrated that a high lipophilicity drug has more difficulty in crossing the hydrophilic mucus layer.
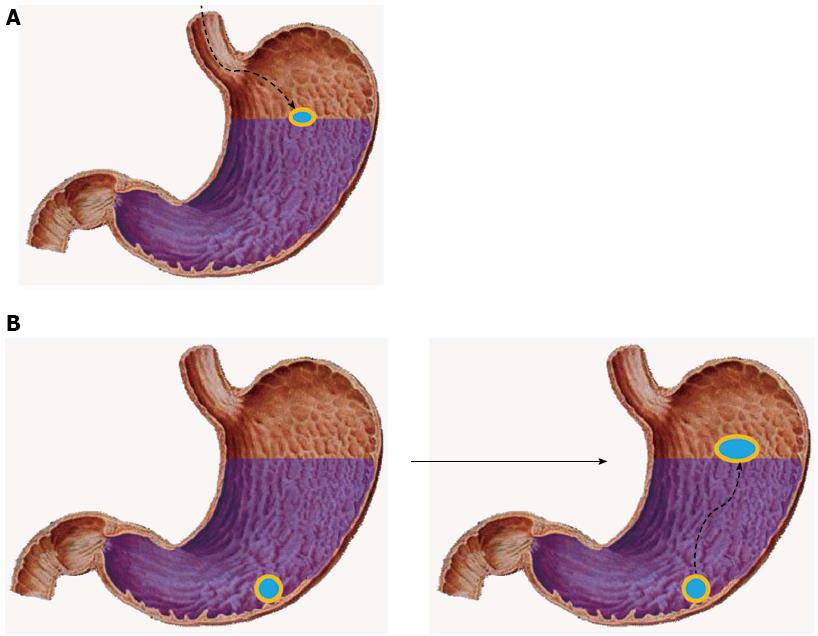
Floating drug delivery systems were first described by Davis in 1968 and are low-density systems that can prolong the gastric retention time and increase bioavailability by floating on top of the gastric contents[58]. The desired formulation of floating systems should comply with the following requirements: (1) maintaining a lower density or higher buoyancy than that of the gastric contents (1.004-1.010); (2) providing a sufficient barrier to protect the stability of the active agent from gastric acid; and (3) releasing the drug in a controlled or sustained manner to achieve the therapeutic effect.
Usually, floating formulations are prepared using hydrophilic matrices that either have a lower density (Figure 1A) or float on the top of the gastric fluid after absorbing water and swelling (Figure 1B). Cellulose ether polymers are used as the hydrophilic matrices, and low-density fatty acids are also used to increase the buoyancy. Hydrodynamically balanced systems (HBSs), gas-generating systems and low-density systems are the primary approaches used in designing intragastric floating systems[59].
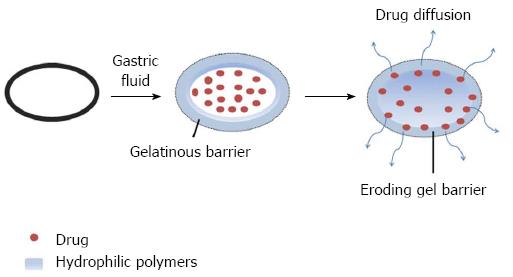
HBSs are a single-unit dosage form containing a mixture of drugs, a gel barrier comprising hydrophilic polymers and other excipients. Hydroxypropyl methyl cellulose, ethylcellulose), hydroxyethylcellulose, hydroxypropylcellulose, sodium carboxymethylcellulose, agar, carrageenansand alginate acid are commonly used excipients. After oral administration, the hydrocolloid is dissolved by either hydration or swelling and maintained at a low density, which allows for controlled drug release from the swollen gel matrix (Figure 2)[6,60].
Gas-generating systems achieve their buoyant properties by the generation of gas bubbles. For example, CO2 can be generated from sodium bicarbonate at an acidic pH. Thus, acids are required in the formulation. These systems include single- and multi-unit forms[61,62].
Low-density systems are made of low-density materials, entrapping oil or air. Multi-unit systems such as microparticles are the most common type[63,64]. The low-density materials that are commonly used include empty hard gelatin capsules, polystyrene foams and pop-rice grains. The external surface of the low-density materials comprises EC and cellulose acetate phthalate and is coated with drugs, with the entrapped oil and air providing the desired buoyancy.
In the last decade, the emulsification solvent evaporation method for the preparation of low density systems achieved tremendous popularity. A large number of studies have employed this method, and different anti-bacterial agents were encapsulated in these systems for the treatment of H. pylori (e.g., metronidazole[65,66], tetracycline[67] and amoxicillin[68]). A number of commercialized floating dosage products exist in the market, one of which targets H. pylori, and they are listed in Table 2[6].
| Product | Remarks | Active ingredient | Company |
| Topalkan | Loating liquid alginate | Aluminum magnesium | Pierre Fabre Medicament, France |
| Almagate flatcoat | Floating liquid form | Antacid | |
| proQuin XR | AcuformTM | Ciprofloxacin | Depomed, United States |
Yang et al[67] prepared a triple layer tablet based on HBSs, which was composed of a rate-controlling polymer matrix and a drug core. Hydroxypropyl methylcellulose and poly (ethylene oxide) comprised the polymer layers, and tetracycline and metronidazole were encapsulated in the core. The in vitro evaluation demonstrated the sustained delivery of the antibiotics over 6-8 h while the tablet remained afloat. Rajinikanth et al[68] developed an intragastric in situ gelling system that floated for the controlled delivery of amoxicillin in the treatment of H. pylori infections. The in vivo H. pylori clearance efficacy was 10 times higher than the effects in an in vivo gerbil model because of the prolonged GI residence time of the formulation.
Bioadhesive/mucoadhesive drug delivery systems are a dosage form that can stick to the mucosal surface by different mechanisms. This formulation involves a mucous coating or an adhesive polymer and is considered to be a bioadhesive form[69]. The adhesive polymers primarily used for bioadhesive materials are listed in Table 3[70]. Micro- and nanoparticles, which have higher mucosal permeability and drug delivery efficiency, are thought to be an ideal bioadhesive carrier[71]. Once this dosage form is orally administered, it is dissolved in the gastric liquid and firmly sticks to the mucosal surface, which is predicted to prolong the residence time of the drug. The electronic theory, adsorption theory, wetting theory and diffusion theory are invoked to explain the adhesive mechanisms[72,73].
Despite the bioadhesive properties of these polymers, they are difficult to maintain because of the turnover of gastric mucosa and gastric emptying. Despite these difficulties, several studies have shown promising results.
Liu et al[81] published a study on amoxicillin bioadhesive microspheres that use an emulsion-solvent evaporation technique, with Carbopol-934p as the mucoadhesive polymer and EC as the matrix. The in vitro release test showed that approximately 90% of the amoxicillin was released in the pH 1.0 HCl solution after 4 h. In vitro and in vivo mucoadhesive tests showed that mucoadhesive microspheres adhered more strongly to the gastric mucous layer than non-adhesive amoxicillin microspheres. Amoxicillin mucoadhesive microspheres were retained in the GI tract for an extended period of time[81]. Jayvadan KP and Jayant RC also formulated mucoadhesive amoxicillin microspheres for the treatment of H. pylori infections. They prepared the microspheres using a similar method and carrier polymer. The amoxicillin microspheres showed a high efficiency of drug entrapment. The in vitro adhesive test showed that mucoadhesive microspheres adhered more strongly to the gastric mucous and that more than 50% of the microspheres were retained in the GI tract after 12 h. Furthermore, in vivo tests were performed by orally administering amoxicillin powder and mucoadhesive microspheres in an H. pylori infection animal model under fed conditions at single and multiple doses. The results indicated that the mucoadhesive microspheres had a better clearance than the powder[82].
Although significant advances have been made in both floating and bioadhesive systems, there are still many challenges. First, the floating systems are unable to release the drug at the intended site. Second, gastric emptying may reduce the buoyancy of the floating systems in the stomach. Third, the turnover of the gastric mucosa and gastric emptying reduce the adhesive force of bioadhesive systems. Therefore, a dual working system would overcome the drawbacks associated with bioadhesive and floating systems and would have a significant effect on improving the therapeutic outcomes.
Zheng et al[83] designed floating-bioadhesive microparticles to increase the efficacy of antibiotics against H. pylori. The formulation containing clarithromycin was prepared by the method of emulsification/evaporation and internal gelation with EC, sodium alginate and chitosan. In vitro buoyancy and drug release tests showed that approximately 74% of the microparticles floated in an acetate buffer solution for 8 h and 90% of the clarithromycin was released in a sustained manner within 8 h. An in vivo mucoadhesive test demonstrated that 61% of the microparticles were retained in the stomach after 4 h. Rajinikanth et al[84] developed stomach-specific floating-bioadhesive microspheres of clarithromycin for the treatment of H. pylori infections. The microspheres were prepared by the emulsification-solvent evaporation method using EC as the matrix polymer and Carbopol-934P as the bioadhesive material. The microspheres showed strong mucoadhesive properties and good buoyancy during the in vitro evaluation and a significant anti-H. pylori effect after oral administration to Mongolian gerbils infected with H. pylori.
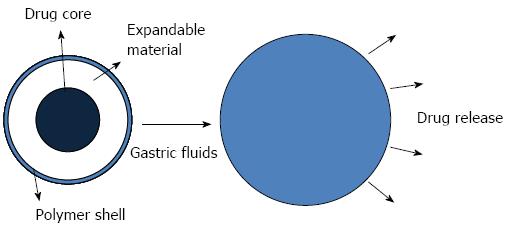
The dosage size in the stomach is one of the influential factors of gastric retention time. Expandable drug delivery systems are small for easy swallowing and expand to a larger size after contact with gastric juices, which can prolong the gastric retention time (Figure 3)[85,86]. Thus, an optimal expandable dosage requires the following properties: convenience for oral ingestion, expandable upon contact with the gastric contents, controlled drug release and either a degradable nature or a reduction in size enabling safe evacuation after drug release[85,87,88]. Superporous hydrogels that are pH and temperature sensitive, are fast-swelling and have a high swelling capacity are considered to be a novel material for swellable systems[89]. Chen et al[90] synthesized superporous hydrogels that used croscarmellose sodium (AC-Di-Sol®) as a composite material, and its addition resulted in a significant improvement in the properties of the superporous hydrogels. Park et al[91] prepared chitosan-based superporous hydrogels using freeze drying and gas blowing techniques. In vitro tests showed that the superporous hydrogels were highly sensitive to the pH of the swelling media. These swelling behaviors and degradation kinetics are important variables in determining the gastric retention time.
Diseases of the GI tract present challenging targets for drug delivery, particularly for oral formulations. Recently, advances in micro- and nanotechnology research opened up a vast potential for the development of GI-targeted drug delivery systems. Liposomes, a type of nanoparticle, became a focal point of GI-targeted delivery systems[92,93]. Liposomes are microscopic phospholipid bubbles with a bilayer membrane[94]. They are nontoxic, nonhemolytic and nonimmunogenic[95]. Singh et al[96] prepared double liposomes that encapsulated two drugs: the inner liposome was first loaded with one drug(amoxicillin) by thin film hydration and then was enveloped with a thin lipid film containing the second drug(ranitidine bismuth citrate). This formulation showed prolonged, sustained drug release, efficient binding to H. pylori, increased inhibition of bacterial growth, reduced bacterial secretion and an ulcer protective ability in an in vitro test. Jain et al[97] designed a gastroretentive polyelectrolyte-coated multilayered liposome containing amoxicillin and metronidazole. The system was prepared by alternative coatings of polyanionic poly (acrylicacid) and polycationic poly (allylamine hydrochloride), using liposomes as the core. Compared with conventional liposomes, the multilayered liposomes showed prolonged drug release in simulated gastric fluid. In vitro and in vivo evaluations indicated the bacterial clearance activity and binding propensity of the system were good.
Over the past two decades, there have been significant advances in the development of gastroretentive drug delivery systems for the treatment of H. pylori infections. The literature has shown that gastroretentive dosage forms are effective at not only prolonging retention time in the stomach but also targeting H. pylori. However, we still lack sufficient in vivo data, especially in humans. Although some studies have indicated that the gastroretentive delivery systems work well in animal models (e.g., rats and Mongolian gerbils), these results may not translate to humans because of the differences among species. Therefore, screening and synthesizing new active agents and developing efficient targeted drug delivery systems are drawing more focus in the field, with one example being ligand-targeted drug formulations based on specific interactions[98,99]. However, with the development and study of natural products, many traditional herb extracts, such as ascardole, Mexicantea herbs and Pterocarpus santalinus, have exhibited anti-H. pylori activity and may serve as potential treatment options[100-102].
Progress in developing an efficient gastroretentive form for the eradication of H. pylori is closely linked to the development of pharmaceutical technology and functional polymer materials and to an increased understanding of the mechanisms of H. pylori pathogenicity.
P- Reviewer: Annalisa G, Handa O S- Editor: Zhai HH L- Editor: A E- Editor: Zhang DN
| 1. | Goh KL, Chan WK, Shiota S, Yamaoka Y. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter. 2011;16 Suppl 1:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 232] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 2. | Gisbert JP. [Helicobacter pylori-related diseases]. Gastroenterol Hepatol. 2012;35 Suppl 1:12-25. [PubMed] [Cited in This Article: ] |
| 3. | Umamaheshwari RB, Jain S, Jain NK. A new approach in gastroretentive drug delivery system using cholestyramine. Drug Deliv. 2003;10:151-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Shah S, Qaqish R, Patel V, Amiji M. Evaluation of the factors influencing stomach-specific delivery of antibacterial agents for Helicobacter pylori infection. J Pharm Pharmacol. 1999;51:667-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Nagahara N, Akiyama Y, Nakao M, Tada M, Kitano M, Ogawa Y. Mucoadhesive microspheres containing amoxicillin for clearance of Helicobacter pylori. Antimicrob Agents Chemother. 1998;42:2492-2494. [PubMed] [Cited in This Article: ] |
| 6. | Pawar VK, Kansal S, Garg G, Awasthi R, Singodia D, Kulkarni GT. Gastroretentive dosage forms: a review with special emphasis on floating drug delivery systems. Drug Deliv. 2011;18:97-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Hwang SJ, Park H, Park K. Gastric retentive drug-delivery systems. Crit Rev Ther Drug Carrier Syst. 1998;15:243-284. [PubMed] [Cited in This Article: ] |
| 8. | Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720-741. [PubMed] [Cited in This Article: ] |
| 9. | Marshall B. Helicobacter pylori: 20 years on. Clin Med. 2002;2:147-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2805] [Cited by in F6Publishing: 2668] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 11. | Yamada T, Ahnen D, Alpers DH, Greenberg HB, Gray L, Joscelyn KB, Kauffman G, Podolsky DK, Ray WA, Schaberg D. Helicobacter-Pylori in Peptic-Ulcer Disease. Jama-J Am Med Assoc. 1994;272:65-69. [DOI] [Cited in This Article: ] [Cited by in Crossref: 756] [Cited by in F6Publishing: 752] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 12. | Singh K, Ghoshal UC. Causal role of Helicobacter pylori infection in gastric cancer: an Asian enigma. World J Gastroenterol. 2006;12:1346-1351. [PubMed] [Cited in This Article: ] |
| 13. | Goodwin CS, Armstrong JA. Microbiological aspects of Helicobacter pylori (Campylobacter pylori). Eur J Clin Microbiol Infect Dis. 1990;9:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 96] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Geis G, Suerbaum S, Forsthoff B, Leying H, Opferkuch W. Ultrastructure and biochemical studies of the flagellar sheath of Helicobacter pylori. J Med Microbiol. 1993;38:371-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Semino-Mora C, Doi SQ, Marty A, Simko V, Carlstedt I, Dubois A. Intracellular and interstitial expression of Helicobacter pylori virulence genes in gastric precancerous intestinal metaplasia and adenocarcinoma. J Infect Dis. 2003;187:1165-1177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Krajewska B. Ureases I. Functional, catalytic and kinetic properties: A review. J Mol Catal B-Enzym. 2009;59:9-21. [DOI] [Cited in This Article: ] [Cited by in Crossref: 483] [Cited by in F6Publishing: 491] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 17. | Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 448] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 18. | Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1446] [Cited by in F6Publishing: 1415] [Article Influence: 78.6] [Reference Citation Analysis (1)] |
| 19. | Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 626] [Cited by in F6Publishing: 656] [Article Influence: 32.8] [Reference Citation Analysis (1)] |
| 20. | Cover TL, Blaser MJ. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570-10575. [PubMed] [Cited in This Article: ] |
| 21. | Guillemin K, Salama NR, Tompkins LS, Falkow S. Cag pathogenicity island-specific responses of gastric epithelial cells to Helicobacter pylori infection. Proc Natl Acad Sci USA. 2002;99:15136-15141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 173] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Genta RM, Graham DY. Helicobacter pylori: the new bug on the (paraffin) block. Virchows Arch. 1994;425:339-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Cid TP, Fernández MC, Benito Martínez S, Jones NL. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2013;18 Suppl 1:12-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Oertli M, Noben M, Engler DB, Semper RP, Reuter S, Maxeiner J, Gerhard M, Taube C, Müller A. Helicobacter pylori γ-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci USA. 2013;110:3047-3052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 25. | Goodgame RW, Malaty HM, el-Zimaity HM, Graham DY. Decrease in gastric permeability to sucrose following cure of Helicobacter pylori infection. Helicobacter. 1997;2:44-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Borch K, Sjöstedt C, Hannestad U, Söderholm JD, Franzén L, Mårdh S. Asymptomatic Helicobacter pylori gastritis is associated with increased sucrose permeability. Dig Dis Sci. 1998;43:749-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Noach LA, Rolf TM, Tytgat GN. Electron microscopic study of association between Helicobacter pylori and gastric and duodenal mucosa. J Clin Pathol. 1994;47:699-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 107] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | McColl KE. Helicobacter pylori and acid secretion: where are we now? Eur J Gastroenterol Hepatol. 1997;9:333-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Go MF, Kapur V, Graham DY, Musser JM. Population genetic analysis of Helicobacter pylori by multilocus enzyme electrophoresis: extensive allelic diversity and recombinational population structure. J Bacteriol. 1996;178:3934-3938. [PubMed] [Cited in This Article: ] |
| 30. | O’Connor A, Gisbert JP, McNamara D, O’Morain C. Treatment of Helicobacter pylori Infection 2011. Helicobacter. 2011;16:6. [Cited in This Article: ] |
| 31. | Cavallaro LG, Egan B, O’Morain C, Di Mario F. Treatment of Helicobacter pylori infection. Helicobacter. 2006;11 Suppl 1:36-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Gumurdulu Y, Serin E, Ozer B, Kayaselcuk F, Ozsahin K, Cosar AM, Gursoy M, Gur G, Yilmaz U, Boyacioglu S. Low eradication rate of Helicobacter pylori with triple 7-14 days and quadriple therapy in Turkey. World J Gastroenterol. 2004;10:668-671. [PubMed] [Cited in This Article: ] |
| 33. | Ogata SK, Godoy AP, da Silva Patricio FR, Kawakami E. High Helicobacter pylori resistance to metronidazole and clarithromycin in Brazilian children and adolescents. J Pediatr Gastroenterol Nutr. 2013;56:645-648. [PubMed] [Cited in This Article: ] |
| 34. | Gomollón F, Santolaria S, Sicilia B, Ferrero M, Revillo MJ, Ducóns J, Villar M, Celaya MC, Montoro M. [Helicobacter pylori resistance to metronidazole and clarythromicin: descriptive analysis 1997-2000]. Med Clin (Barc). 2004;123:481-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Mégraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53:1374-1384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 607] [Cited by in F6Publishing: 653] [Article Influence: 32.7] [Reference Citation Analysis (1)] |
| 36. | Seddik H, Ahid S, El Adioui T, El Hamdi FZ, Hassar M, Abouqal R, Cherrah Y, Benkirane A. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a prospective randomized study. Eur J Clin Pharmacol. 2013;69:1709-1715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Lim JH, Lee DH, Choi C, Lee ST, Kim N, Jeong SH, Kim JW, Hwang JH, Park YS, Lee SH. Clinical outcomes of two-week sequential and concomitant therapies for Helicobacter pylori eradication: a randomized pilot study. Helicobacter. 2013;18:180-186. [PubMed] [Cited in This Article: ] |
| 38. | Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 39. | Tsay FW, Tseng HH, Hsu PI, Wang KM, Lee CC, Chang SN, Wang HM, Yu HC, Chen WC, Peng NJ. Sequential therapy achieves a higher eradication rate than standard triple therapy in Taiwan. J Gastroenterol Hepatol. 2012;27:498-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Molina-Infante J, Romano M, Fernandez-Bermejo M, Federico A, Gravina AG, Pozzati L, Garcia-Abadia E, Vinagre-Rodriguez G, Martinez-Alcala C, Hernandez-Alonso M. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology. 2013;145:121-128.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 41. | Molina-Infante J, Pazos-Pacheco C, Vinagre-Rodriguez G, Perez-Gallardo B, Dueñas-Sadornil C, Hernandez-Alonso M, Gonzalez-Garcia G, Mateos-Rodriguez JM, Fernandez-Bermejo M, Gisbert JP. Nonbismuth quadruple (concomitant) therapy: empirical and tailored efficacy versus standard triple therapy for clarithromycin-susceptible Helicobacter pylori and versus sequential therapy for clarithromycin-resistant strains. Helicobacter. 2012;17:269-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 42. | Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011;16:139-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 43. | Kuo CH, Kuo FC, Hu HM, Liu CJ, Wang SS, Chen YH, Hsieh MC, Hou MF, Wu DC. The Optimal First-Line Therapy of Helicobacter pylori Infection in Year 2012. Gastroenterol Res Pract. 2012;2012:168361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Sardarian H, Fakheri H, Hosseini V, Taghvaei T, Maleki I, Mokhtare M. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter. 2013;18:129-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 45. | Bardonnet PL, Faivre V, Pugh WJ, Piffaretti JC, Falson F. Gastroretentive dosage forms: overview and special case of Helicobacter pylori. J Control Release. 2006;111:1-18. [PubMed] [Cited in This Article: ] |
| 46. | Strid H, Simrén M, Stotzer PO, Abrahamsson H, Björnsson ES. Delay in gastric emptying in patients with chronic renal failure. Scand J Gastroenterol. 2004;39:516-520. [PubMed] [Cited in This Article: ] |
| 47. | Horowitz M, Wishart JM, Jones KL, Hebbard GS. Gastric emptying in diabetes: an overview. Diabet Med. 1996;13:S16-S22. [PubMed] [Cited in This Article: ] |
| 48. | Leontiadis GI, Minopoulos GI, Maltezos E, Kotsiou S, Manolas KI, Simopoulos K, Hatseras D. Effects of Helicobacter pylori infection on gastric emptying rate in patients with non-ulcer dyspepsia. World J Gastroenterol. 2004;10:1750-1754. [PubMed] [Cited in This Article: ] |
| 49. | McHugh PR, Moran TH. Calories and gastric emptying: a regulatory capacity with implications for feeding. Am J Physiol. 1979;236:R254-R260. [PubMed] [Cited in This Article: ] |
| 50. | Kwiatek MA, Menne D, Steingoetter A, Goetze O, Forras-Kaufman Z, Kaufman E, Fruehauf H, Boesiger P, Fried M, Schwizer W. Effect of meal volume and calorie load on postprandial gastric function and emptying: studies under physiological conditions by combined fiber-optic pressure measurement and MRI. Am J Physiol Gastrointest Liver Physiol. 2009;297:G894-G901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 51. | Hunt JN, Stubbs DF. The volume and energy content of meals as determinants of gastric emptying. J Physiol. 1975;245:209-225. [PubMed] [Cited in This Article: ] |
| 52. | Kaniwa N, Aoyagi N, Ogata H, Ejima A. Gastric emptying rates of drug preparations. I. Effects of size of dosage forms, food and species on gastric emptying rates. J Pharmacobiodyn. 1988;11:563-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Timmermans J, Moës AJ. The cutoff size for gastric emptying of dosage forms. J Pharm Sci. 1993;82:854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Khosla R, Davis SS. The Effect of Tablet Size on the Gastric-Emptying of Nondisintegrating Tablets. Inter J Pharm. 1990;62:R9-R11. [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Coupe AJ, Davis SS, Evans DF, Wilding IR. Correlation of the gastric emptying of nondisintegrating tablets with gastrointestinal motility. Pharm Res. 1991;8:1281-1285. [PubMed] [Cited in This Article: ] |
| 56. | Sigurdsson HH, Kirch J, Lehr CM. Mucus as a barrier to lipophilic drugs. Int J Pharm. 2013;453:56-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 57. | Larhed AW, Artursson P, Gråsjö J, Björk E. Diffusion of drugs in native and purified gastrointestinal mucus. J Pharm Sci. 1997;86:660-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 128] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 58. | Getyala A, Gangadharappa HV, Prasad MS, Reddy MP, Kumar TM. Formulation and evaluation of non-effervescent floating tablets of losartan potassium. Curr Drug Deliv. 2013;10:620-629. [PubMed] [Cited in This Article: ] |
| 59. | Singh BN, Kim KH. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release. 2000;63:235-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 356] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 60. | Wettrell K, Pandolfi M. Effect of oral administration of various beta-blocking agents on the intraocular pressure in healthy volunteers. Exp Eye Res. 1975;21:451-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Wei Z, Yu Z, Bi D. Design and evaluation of a two-layer floating tablet for gastric retention using cisapride as a model drug. Drug Dev Ind Pharm. 2001;27:469-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Chen YC, Lee LW, Ho HO, Sha C, Sheu MT. Evaluation of water uptake and mechanical properties of blended polymer films for preparing gas-generated multiple-unit floating drug delivery systems. J Pharm Sci. 2012;101:3811-3822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Prinderre P, Sauzet C, Fuxen C. Advances in gastro retentive drug-delivery systems. Expert Opin Drug Deliv. 2011;8:1189-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Gröning R, Cloer C, Georgarakis M, Müller RS. Compressed collagen sponges as gastroretentive dosage forms: in vitro and in vivo studies. Eur J Pharm Sci. 2007;30:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Ishak RA, Awad GA, Mortada ND, Nour SA. Preparation, in vitro and in vivo evaluation of stomach-specific metronidazole-loaded alginate beads as local anti-Helicobacter pylori therapy. J Control Release. 2007;119:207-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 66. | Sriamornsak P, Thirawong N, Puttipipatkhachorn S. Emulsion gel beads of calcium pectinate capable of floating on the gastric fluid: effect of some additives, hardening agent or coating on release behavior of metronidazole. Eur J Pharm Sci. 2005;24:363-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 67. | Yang L, Eshraghi J, Fassihi R. A new intragastric delivery system for the treatment of Helicobacter pylori associated gastric ulcer: in vitro evaluation. J Control Release. 1999;57:215-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 68. | Rajinikanth PS, Balasubramaniam J, Mishra B. Development and evaluation of a novel floating in situ gelling system of amoxicillin for eradication of Helicobacter pylori. Int J Pharm. 2007;335:114-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 69. | Vasir JK, Tambwekar K, Garg S. Bioadhesive microspheres as a controlled drug delivery system. Int J Pharm. 2003;255:13-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 322] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 70. | Talukder R, Fassihi R. Gastroretentive delivery systems: a mini review. Drug Dev Ind Pharm. 2004;30:1019-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 71. | Ponchel G, Irache J. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv Drug Deliv Rev. 1998;34:191-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 324] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 72. | Huang Y, Leobandung W, Foss A, Peppas NA. Molecular aspects of muco- and bioadhesion: tethered structures and site-specific surfaces. J Control Release. 2000;65:63-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 179] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 73. | Park K, Robinson JR. Bioadhesive Polymers as Platforms for Oral-Controlled Drug Delivery - Method to Study Bioadhesion. Inter J Pharm. 1984;19:107-127. [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 265] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 74. | Rastogi R, Sultana Y, Aqil M, Ali A, Kumar S, Chuttani K, Mishra AK. Alginate microspheres of isoniazid for oral sustained drug delivery. Int J Pharm. 2007;334:71-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 75. | Leucuta SE, Ponchel G, Duchêne D. Oxprenolol release from bioadhesive gelatin/poly(acrylic acid) microspheres. J Microencapsul. 1997;14:511-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 76. | Jimenezcastellanos MR, Zia H, Rhodes CT. Mucoadhesive Drug Delivery Systems. Drug Dev Ind Pharm. 1993;19:143-194. [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 77. | Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, Fairbrother DH, Cascio B, Elisseeff JH. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 2007;6:385-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 537] [Cited by in F6Publishing: 445] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 78. | Raval JA, Patel MM. Formulation and Characterization of Gastroretentive Discs Containing Famotidine. B. raz Arch BiolTechn. 2011;54:293-300. [DOI] [Cited in This Article: ] |
| 79. | Hejazi R, Amiji M. Chitosan-based gastrointestinal delivery systems. J Control Release. 2003;89:151-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 611] [Cited by in F6Publishing: 621] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 80. | Zou W, Cao G, Xi Y, Zhang N. New approach for local delivery of rapamycin by bioadhesive PLGA-carbopol nanoparticles. Drug Deliv. 2009;16:15-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 81. | Liu Z, Lu W, Qian L, Zhang X, Zeng P, Pan J. In vitro and in vivo studies on mucoadhesive microspheres of amoxicillin. J Control Release. 2005;102:135-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Patel JK, Chavda JR. Formulation and evaluation of stomach-specific amoxicillin-loaded carbopol-934P mucoadhesive microspheres for anti-Helicobacter pylori therapy. J Microencapsul. 2009;26:365-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | Zheng JH, Liu CW, Bao DC, Zhao YJ, Ma XJ. Preparation and evaluation of floating-bioadhesive microparticles containing clarithromycin for the eradication of Helicobacter pylori. J Appl Polym Sci. 2006;102:2226-2232. [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Rajinikanth PS, Karunagaran LN, Balasubramaniam J, Mishra B. Formulation and evaluation of clarithromycin microspheres for eradication of Helicobacter pylori. Chem Pharm Bull (Tokyo). 2008;56:1658-1664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | Klausner EA, Lavy E, Friedman M, Hoffman A. Expandable gastroretentive dosage forms. J Control Release. 2003;90:143-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 86. | Nayak AK, Malakar J, Sen KK. Gastroretentive drug delivery technologies: current approaches and future potential. J Pharm Educ Res. 2010;1:12. [Cited in This Article: ] |
| 87. | Gröning R, Cloer C, Müller RS. Development and in vitro evaluation of expandable gastroretentive dosage forms based on compressed collagen sponges. Pharmazie. 2006;61:608-612. [PubMed] [Cited in This Article: ] |
| 88. | Matharu AS, Motto MG, Patel MR, Simonelli AP, Dave RH. Evaluation of hydroxypropyl methylcellulose matrix systems as swellable gastro-retentive drug delivery systems (GRDDS). J Pharm Sci. 2011;100:150-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 89. | Mastropietro DJ, Omidian H, Park K. Drug delivery applications for superporous hydrogels. Expert Opin Drug Deliv. 2012;9:71-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Chen J, Blevins WE, Park H, Park K. Gastric retention properties of superporous hydrogel composites. J Control Release. 2000;64:39-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 164] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 91. | Park H, Park K, Kim D. Preparation and swelling behavior of chitosan-based superporous hydrogels for gastric retention application. J Biomed Mater Res A. 2006;76:144-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 92. | Zhang JX, Wang K, Mao ZF, Fan X, Jiang DL, Chen M, Cui L, Sun K, Dang SC. Application of liposomes in drug development--focus on gastroenterological targets. Int J Nanomedicine. 2013;8:1325-1334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 93. | Freund O. Biodistribution and gastrointestinal drug delivery of new lipidic multilamellar vesicles. Drug Deliv. 2001;8:239-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 94. | Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3592] [Cited by in F6Publishing: 3300] [Article Influence: 173.7] [Reference Citation Analysis (0)] |
| 95. | Gregoriadis G, Florence AT. Liposomes in drug delivery. Clinical, diagnostic and ophthalmic potential. Drugs. 1993;45:15-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 244] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 96. | Singh DY, Prasad NK. Double liposomes mediated dual drug targeting for treatment of Helicobacter pylori infections. Pharmazie. 2011;66:368-373. [PubMed] [Cited in This Article: ] |
| 97. | Jain P, Jain S, Prasad KN, Jain SK, Vyas SP. Polyelectrolyte coated multilayered liposomes (nanocapsules) for the treatment of Helicobacter pylori infection. Mol Pharm. 2009;6:593-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | Lin YH, Tsai SC, Lai CH, Lee CH, He ZS, Tseng GC. Genipin-cross-linked fucose-chitosan/heparin nanoparticles for the eradication of Helicobacter pylori. Biomaterials. 2013;34:4466-4479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 99. | Ramteke S, Ganesh N, Bhattacharya S, Jain NK. Amoxicillin, clarithromycin, and omeprazole based targeted nanoparticles for the treatment of H. pylori. J Drug Target. 2009;17:225-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 100. | Cao MB, Dong L, Chang XM, Zou BC, Qin B. Effect of Mexican tea herb and pilular adina herb on concrescence of gastric mucosa in experimental gastric ulcer rats. Chin J Integr Med. 2007;13:132-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 101. | Narayan S, Veeraraghavan M, Devi CS. Pterocarpus santalinus: an In Vitro study on its anti-Helicobacter pylori effect. Phytother Res. 2007;21:190-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 102. | Wang YC, Huang TL. Screening of anti-Helicobacter pylori herbs deriving from Taiwanese folk medicinal plants. FEMS Immunol Med Microbiol. 2005;43:295-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |