Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Jul 14, 2014; 20(26): 8722-8725
Published online Jul 14, 2014. doi: 10.3748/wjg.v20.i26.8722
Published online Jul 14, 2014. doi: 10.3748/wjg.v20.i26.8722
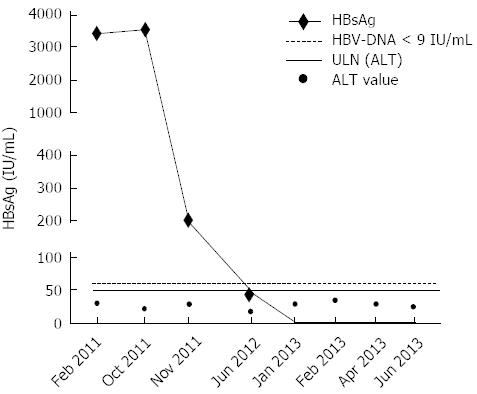
Figure 2 HBsAg, hepatitis B virus-DNA and alanine aminotransferase serum level assessment.
A decrease of the starting HBsAg titre (3533 IU/mL) was observed 1 mo after the beginning of Peg-interferon α-2a (1.21 log; HBsAg titre 218 IU/mL) and 3 mo after the discontinuation of all drugs (1.88 log; HBsAg titre 47 IU/mL). Seven months later, the quantitative determination of HBsAg resulted negative. The patient showed steadily undetectable HBV-DNA (< 9 IU/mL) and normal ALT levels (< ULN). IU: International unit; ULN: Upper limit of normal.
- Citation: Barone M, Iannone A, Leo AD. HBsAg clearance by Peg-interferon addition to a long-term nucleos(t)ide analogue therapy. World J Gastroenterol 2014; 20(26): 8722-8725
- URL: https://www.wjgnet.com/1007-9327/full/v20/i26/8722.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i26.8722