Published online Jul 14, 2014. doi: 10.3748/wjg.v20.i26.8416
Revised: January 30, 2014
Accepted: March 12, 2014
Published online: July 14, 2014
Processing time: 252 Days and 5.7 Hours
Iron deficiency anemia (IDA) is common and often under recognized problem in the elderly. It may be the result of multiple factors including a bleeding lesion in the gastrointestinal tract. Twenty percent of elderly patients with IDA have a negative upper and lower endoscopy and two-thirds of these have a lesion in the small bowel (SB). Capsule endoscopy (CE) provides direct visualization of entire SB mucosa, which was not possible before. It is superior to push enteroscopy, enteroclysis and barium radiography for diagnosing clinically significant SB pathology resulting in IDA. Angioectasia is one of the commonest lesions seen on the CE in elderly with IDA. The diagnostic yield of CE for IDA progressively increases with advancing age, and is highest among patients over 85 years of age. Balloon assisted enteroscopy is used to treat the lesions seen on CE. CE has some limitations mainly lack of therapeutic capability, inability to provide precise location of the lesion and false positive results. Overall CE is a very safe and effective procedure for the evaluation of IDA in elderly.
Core tip: Iron deficiency anemia (IDA) is a common problem especially in the elderly. Small bowel (SB) lesions may be the source of IDA. Capsule endoscopy (CE) provides direct visualization of entire SB mucosa. Angioectasia is one of the commonest lesions seen on the CE in elderly with IDA. The diagnostic yield of CE for IDA increases with advancing age. Balloon assisted enteroscopy is used to treat the lesions seen on CE causing IDA.
- Citation: Muhammad A, Vidyarthi G, Brady P. Role of small bowel capsule endoscopy in the diagnosis and management of iron deficiency anemia in elderly: A comprehensive review of the current literature. World J Gastroenterol 2014; 20(26): 8416-8423
- URL: https://www.wjgnet.com/1007-9327/full/v20/i26/8416.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i26.8416
Iron-deficiency anemia (IDA) is the most common cause of anemia worldwide, causing significant disease-related morbidity, and has a negative impact on patient’s well-being and overall outcome. Anemia is defined by the World Health Organization (WHO) as a hemoglobin concentration of less than 13 mg/dL in men and less than 12 mg/dL in women[1]. The gold standard for the diagnosis of iron deficiency is the absence of iron staining (Prussian blue stain) on bone marrow biopsy. In clinical practice, this invasive test is replaced by evaluation with more readily available laboratory parameters. The classic hallmarks of IDA are low serum ferritin (< 20 ng/L), low serum iron (< 33 g/dL), high serum total iron-binding capacity (> 400 g/dL) and low mean corpuscular volume (< MCV 80 fL).
In the year 2010, 40.3 million people (13.0% of the total population in United States) were 65 years of age and older (elderly), and 5.5 million were above 85 years of age. The elderly population is expected to increase to more than 20% of the total population by 2030, with individuals 85 years and older representing the fastest growing segment of this group. The Third National Health and Nutrition Examination Survey (NHANES III, 1988 to 1994) indicated that the prevalence of IDA was nearly 10.6% in elderly. This means almost 4 million elderly Americans have IDA[2].
IDA is common and often under recognized problem especially in elderly with increased morbidity and mortality. Anemia, however, is not simply a consequence of aging, but also a marker of underlying disease, requiring investigation for an etiology. Anemia is recognized to be associated with increased frailty, poor exercise, performance, diminished cognitive function, dementia, decreased mobility, increased risk for falls, lower bone and muscle density, depression and delirium[3,4]. Anemia is a marker for increased disease-related morbidity, including hospitalization and mortality in elderly[5].
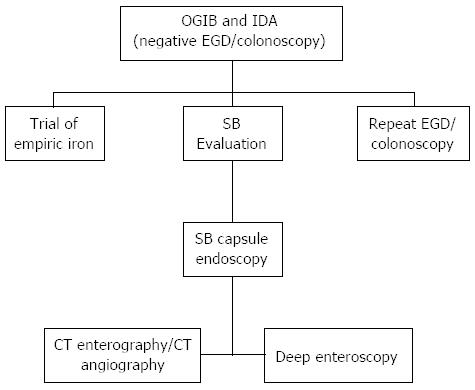
IDA in elderly may be the result of multiple factors. Major considerations include iron deficiency secondary to a bleeding lesion in the gastrointestinal (GI) tract and this is one of the major indications for referral to gastroenterologists (13% of referrals). In clinical practice esophagogastroduodenoscopy (EGD) and colonoscopy are performed in the initial evaluation of IDA to exclude a source of chronic blood loss from the GI tract. Despite undergoing standard endoscopic evaluation, up to 30% of patients with IDA have no definitive diagnosis[6]. Twenty percent of elderly patients have a negative upper and lower endoscopy and two-thirds of them have a lesion in the small bowel (SB)[7]. It is important to investigate the SB in all patients with unexplained IDA, after negative standard dual endoscopic evaluations (ASGE Practice Guideline 2010)[8] (Figure 1).
The original capsule endoscope (PillCam SB) was developed by Given Imaging in 2001[9]. It is a disposable capsule, 11 by 26 mm in size and weighs 3.7 g. There is a set of short focal length lenses located in front of the camera to collect an image in a 140 degree field of view and magnify it by a factor of 8. This optical system provides a resolution of 0.1 mm. The capsule communicates with the external world through a radio transmitter. Each second two pictures are transmitted at 432 MHz to an array of sensors taped to the patient’s abdomen. These sensors in turn relay information to a data recording device worn on a belt. The capsule is powered by two silver oxide batteries with a battery life of 8 h. The new capsule (PillCam SB 3) captures up to 6 frames per second with a 30% improvement in picture resolution.
Since the initial presentation at DDW of 2000, the fantastic voyage of capsule endoscopy (CE) has made significant strides[10]. One of the most significant impacts of the CE has been in the elderly, where it provides a less invasive and virtually complete exam. It has expanded our area of visual survey to include direct visualization of entire SB mucosa, which was not possible before. CE had emerged as a ‘‘light in the darkness’’ for the identification and localization of SB mucosal diseases[9,11]. It has developed an important role in the investigation of patients with IDA when EGD and colonoscopy are negative.
There are a number of other diagnostic modalities, old and new to evaluate the SB, but they all have limitations. CE has made fluoroscopic imaging of SB almost obsolete[12]. Traditional SB X-ray series have the lowest yield and fail to detect many mucosal lesions. CE is superior to SB enteroclysis for detecting lesions in patients with unexplained IDA and should be the next diagnostic test of choice after unremarkable standard endoscopic evaluation[13].
Although push enteroscopy (PE) offers direct visual inspection of the SB mucosa beyond the reach of the standard upper endoscopes, it reaches only 80-120 cm beyond the ligament of Treitz and its sensitivity in identifying the source of bleeding is limited[14]. CE is superior to PE and SB barium radiography for diagnosing clinically significant SB pathology in patients with IDA[15].
CE is performed as per the standard protocols endorsed by American Society for Gastrointestinal Endoscopy (ASGE)[16]. An informed consent is obtained prior to the procedure. Patients usually present in an out-patient setting, after fasting for 8 h. A bowel preparation is optional. They swallow the capsule with few sips of water in a sitting position. A clear liquid breakfast after 2 h and a light meal after 4 h are permitted. After 8 h, patient returns to the endoscopy unit, the data recorder is removed and images are downloaded to the computer. The recordings are then viewed by an experienced reader.
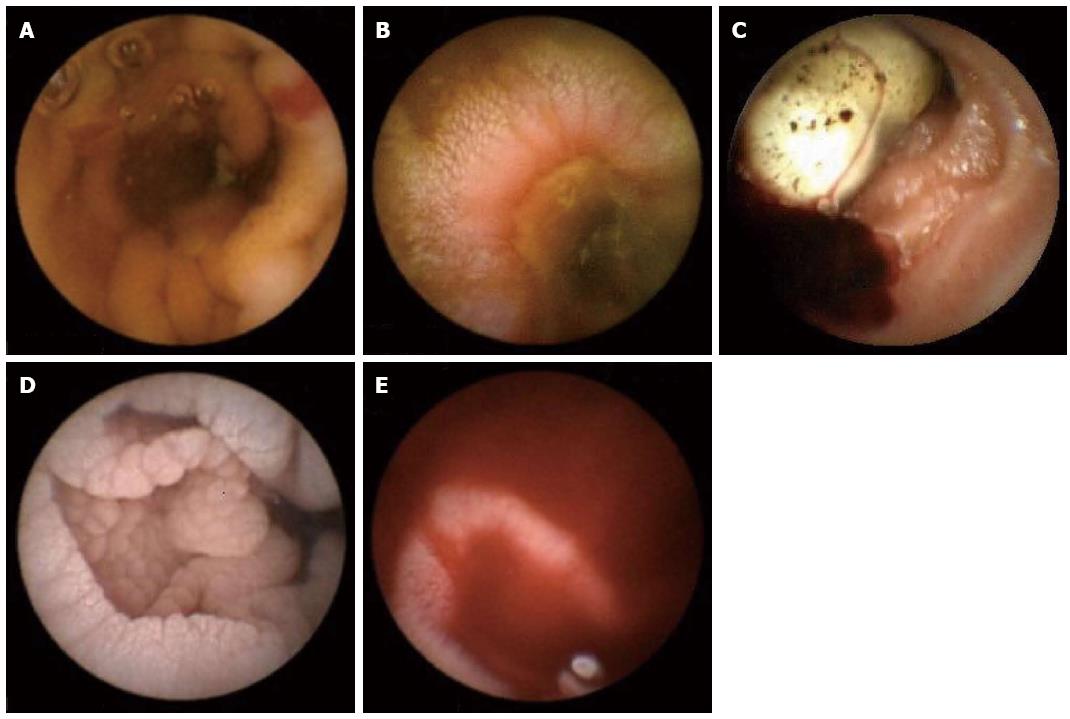
The common SB findings seen on CE when performed for IDA in elderly are[17]: (1) Angioectasia or arterio-venous malformation (Figure 2A); (2) Ulceration [related to nonsteroidal anti-inflammatory drugs (NSAIDs) or Crohn’s disease] (Figure 2B); (3) Tumor or mass lesion (Figure 2C); (4) Celiac disease (CD) (mosaic pattern and scalloping) (Figure 2D); and (5) Active bleeding (Figure 2E).
Angioectasia is one of the commonest found lesion seen on the CE in elderly with IDA. It accounts for 40% of cases of bleeding of obscure origin[18,19] It is seen more commonly in patients with hereditary hemorrhagic telangiectasia, renal disease and in elderly patients with multiple comorbidities[20]. The incidence of angioectasia is about 23%[21]. It varies from a flat lesion greater in diameter than one villus to a pulsatile red protrusion with surrounding venous dilation. They can be treated with argon plasma coagulation or multipolar electrocautery at the time of enteroscopy.
Another common finding, which causes IDA in elderly is the inflammation induced by NSAIDS. The mechanism of action of NSAIDS is COX-1 inhibition and prostaglandin depletion. The Spectrum of NSAID induced SB injury became clear only after the advent of CE, as before 2000 this knowledge was available only from case reports and autopsy series. NSAID induces ulcers may be single or multiple. NSAID induced strictures can be a cause of capsule retention if the stricture was not suspected before administering the capsule. The differential of NSAID induced ulceration includes Crohn’s disease, CD (ulcerative jejuno-ileitis), infection (Cytomegalovirus, Tuberculosis, Yersinia), radiation, ischemia, vasculitis and chemotherapy (mucositis)[22].
CD is another condition where the diagnosis is frequently missed. Because of the increased prevalence of undiagnosed CD (iceberg effect), any test that has a promise of increased detection is of great interest. IDA may be the only manifestation of CD in elderly. Another utility of CE in elderly with CD lies in patient population with refractory CD to evaluate for malignancy such as lymphoma, carcinoma and GISTs. SB tumors are a relatively uncommon diagnosis and account for 3% of all GI malignancies. After gastrointestinal stromal tumor (GIST), adenocarcinoma of the SB is the most common malignancy found in the elderly. In a study of 5129 patients, 129 (2.4%) were found to have SB malignancy[23]. Current data suggests that CE can shorten the diagnostic work up for SB tumor and can influence further management and outcome.
Given the second peak of Crohn’s disease in the elderly, CE can play an important role in the diagnosis of this condition. CE can detect minor (distorted villi, erosion or scars) or major changes (ulcers or strictures) related to Crohn’s disease[24].
The DY of CE in the evaluation of unexplained IDA progressively increases with advancing age, and is highest among patients over 85 years of age. This may be explained by the fact that ASA, NSAIDs, and warfarin usage is more common in elderly as compared with younger patients. Elderly patients with high diagnostic yield (DY) for IDA are likely to benefit more from CE than younger patients. IDA in the elderly with or without obscure GI bleeding (OGIB) is the major indication for CE after a negative EGD and colonoscopy.
There have been a numbers of papers written on the DY of CE when performed for IDA (Table 1). However, there are only few studies which specifically reported on the DY in elderly, and have shown that it is significantly higher as compared to younger age group. Sidhu et al[25] performed a retrospective review of 779 consecutive patients that underwent CE over a 7-year period (2002-2009) for OGIB and recurrent IDA. The DY of CE in elderly was 53%. The most common diagnosis in the elderly was angioectasia (34%). When compared to younger patients, the DY was significantly higher in elderly for IDA (51% vs 37%, P = 0.003, OR = 1.8, 95%CI: 1.3-2.5). Management was also altered in a significant greater proportion in the elderly (P = 0.002, OR = 1.8, 95%CI: 1.3-2.5). The authors finally concluded that CE has a positive impact on the management of IDA in elderly and there should be no barrier to performing CE in this age group. The authors of this study published another paper recently where they reported the DY of CE for IDA is significantly higher in patients who were aged 70 years or older (P < 0.001, OR = 1.9, 95%CI: 1.4-2.6)[26].
| Ref. | Total patients | Patients with positive findings (DY) | Angioectasia | Inflammatory lesions (erosions/ulcers) | Mass lesions | Active bleeding | Other findings (celiac disease, Crohn’s disease) |
| Holleran et al[46] | 65 | 35 (53) | 17 (49) | 12 (34) | 4 (11) | 2 (6) | |
| Sidhu et al[26] | 586 | 245 (42) | 141 (58) | 16 (11) | |||
| Tong et al[47] | 97 | 25 (26) | 6 (24) | 16 (64) | 2 (8) | 1 (4) | |
| Koulaouzidis et al[48] | 221 | 68 (31) | 49 (72) | 19 (28) | |||
| Yamada et al[49] | 30 | 19 (63) | 6 (20) | 7 (37) | 2 (12) | 4 (21) | |
| Efthymiou et al[50] | 40 | 15 (38) | |||||
| Milano et al[51] | 45 | 35 (78) | 13 (37) | 9 (26) | 6 (17) | 7 (20) | |
| Goenka et al[52] | 96 | 35 (37) | |||||
| Katsinelos et al[53] | 38 | 13 (34) | 6 (46) | 4 (31) | 1 (8) | 2 (15) | |
| Riccione et al[54] | 138 | 91 (66) | 51 (56) | 18 (20) | 9 (10) | 13 (14) | |
| Van Turenhourt et al[55] | 240 | 106 (44) | |||||
| Laine et al[56] | 40 | 13 (33) | 4 (31) | 9 (69) | 0 | 0 | |
| Sheibani et al[57] | 57 | 35 (61) | 21 (60) | 4 (12) | 5 (14) | 5 (14) | |
| Kim et al[58] | 25 | 12 (48) | 8 (66) | 2 (17) | 0 | 2 (17) | |
| Sidhu et al[25] | 316 | 152 (48) | 84 (56) | 25 (16) | 10 (6) | 33 (22) | |
| Muhammad et al[27] | 231 | 127 (55) | 35 (28) | 64 (50) | 0 | 15 (12) | 13 (10) |
| Chami et al[59] | 12 | 4 (33) | |||||
| Carey et al[60] | 134 | 62 (46) | 35 (56) | 16 (26) | 4 (6) | 7 (12) | |
| Apostolopoulos et al[61] | 51 | 29 (57) | 12 (41) | 13 (45) | 4 (14) | 0 | |
| Estevez et al[62] | 48 | 30 (63) | |||||
| Van Tuyl et al[63] | 150 | 49 (33) | |||||
| Qvigstaad et al[64] | 40 | 11 (28) | |||||
| Kalantzis et al[65] | 64 | 27 (42) | |||||
| De Leusse et al[66] | 20 | 6 (30) | |||||
| Ben Soussan et al[67] | 18 | 7 (38) | |||||
| Enns et al[68] | 14 | 7 (50) | 2 (29) | 3 (42) | 2 (29) | 0 | |
| Fireman et al[69] | 70 | 37 (52) | 18 (49) | 11 (30) | 0 | 8 (21) | |
| Pennazio et al[17] | 43 | 19 (44) | 4 (21) | 9 (47) | 0 | 6 (32) |
Similarly, we have also published on the DY of CE for IDA which was found to be 69% among patients of > 85 years of age and 56% for the age group of 65-85 years[27]. In another study, we have reported on the utility of CE in the diagnosis of CD in elderly for the first time, when they presented for IDA. We found that out of 279 elderly patients with IDA, 7 (2.5%) had mucosal abnormalities suggestive of CD (atrophy, scalloping, mosaic pattern, layering, and nonspecific ulcerating jejuno-ileitis). Subsequent evaluation with serum antibody testing +/- multiple distal duodenal biopsies confirmed the diagnosis in all patients[28].
Recently Koulaouzidis et al[29] published a systematic review on the DY of CE for IDA in all age groups. The pooled DY of CE in studies focused solely on patients with IDA was 66.6% (95%CI: 61.0%-72.3%).
CE has a number of advantages compared with deep enteroscopy especially in elderly patients. These include the potential ability to visualize the entire SB with a non-invasive procedure carrying a minimal risk of complications, a high sensitivity for lesions and high patient acceptance. These advantages make CE the preferred initial procedure for evaluation of IDA. However, it also has a number of disadvantages including the lack of biopsy and therapeutic capability, the potential for retention above a stricture, and the potential for missed lesions. The overall miss rate for CE is estimated to be 10%-30% in patients with OGIB, and solitary lesions are more likely to be missed[30]. Deep enteroscopy is needed in patients with continued IDA despite a negative CE, and for biopsy and therapy of lesions demonstrated by CE.
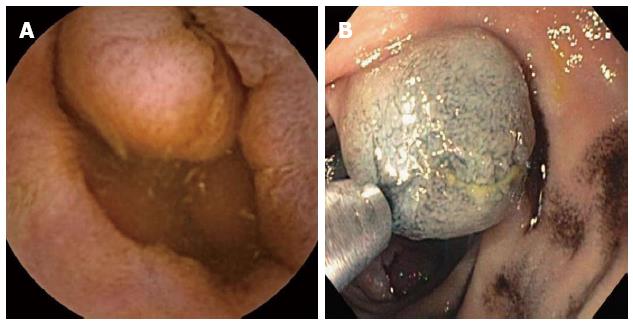
The currently available types of enteroscopy include PE, intra-operative enteroscopy (IOE), balloon assisted enteroscopy (BAE), and spiral enteroscopy (SE). PE has limited applicability because only the duodenum and proximal jejunum can be visualized. IOE has the capability of visualizing the entire SB but carries a significant operative morbidity, and a mortality of up to 4%[31]. SE is a promising new technique which allows deep enteroscopy in a shorter period of time than BAE. This shortens anesthesia time which may be a significant advantage in the elderly. However, it is not widely available, and the system is not currently being sold while modifications to enhance its utility are being developed. This leaves BAE as the most viable option for deep enteroscopy. The greatest advantage of BAE is the ability to biopsy and tattoo the lesion seen on the CE (Figure 3). Other advantages include the therapeutic benefit which includes hemostasis, polypectomy, balloon dilation of strictures and foreign body removal. The disadvantages are that it is invasive with higher risks for complications, time consuming, requires sedation and the entire SB is not visualized in one procedure. When used by both oral and anal route in a given patient, it has the potential to visualize the entire SB.
In two prospective randomized studies of double balloon enteroscopy (DBE) vs single balloon enteroscopy, total enteroscopy was achieved more frequently with DBE. However, there was no difference in DY or therapeutic outcome between the two techniques[32-34]. BAE is frequently a long procedure lasting 60 min or more, and requires deep sedation. Elderly patients may be at increased risk for complications due to prolonged endoscopic procedures, and prolonged sedation due to co-morbidities such as cardiac and pulmonary disease. In addition they are at greater risk of aspiration, have an increased response to sedatives, and have a blunted response to hypoxia and hypercarbia[35]. The complications of BAE include perforation, pancreatitis, and GI bleeding. These occur in 1%-2% of patients which is a higher complication rate compared with standard endoscopic procedures (EGD and colonoscopy)[36,37]. There are only few studies reported on the safety and efficacy of BAE in elderly patients. In a single center, retrospective study on the efficacy and safety of DBE, a total of 60 patients older than 75 years with 110 younger patients were evaluated[38]. Elderly patients were more likely to have angioectasia (39% vs 23%, P = 0.01), and to require endoscopic therapy (48.6% vs 29.2%, P = 0.01). The overall complication rate was 0.9% with no serious complications. There was no difference in the success and complication rates in both age groups in this study.
In another retrospective study, 137 patients (80 years or older) underwent DBE after CE. The correlation between the findings on CE and DBE occurred in 78.9% of patients. False negative findings on CE were present in 6 patients with false positive findings in 18 patients. The DY for DBE in patients with OGIB was 90.2%. No complications related to DBE were encountered in these octogenarians in the 48 h following the procedure. The authors finally concluded that DBE was safe in octogenarians, and that elderly patients with positive findings on CE should be considered for DBE[39].
The majority of studies have reported a good correlation between the findings at capsule endoscopy and BAE and a comparable yield of positive findings[40]. In general, they can be considered “complementary studies”. BAE may be contraindicated in some elderly patients with severe cardiopulmonary co-morbidities. For these reasons, CE should be the first investigation in elderly patients with IDA and other suspected disorders requiring endoscopic SB visualization. BAE is needed for therapy, biopsy and marking of lesions requiring surgery.
A cost-effectiveness analysis for diagnosis of IDA in elderly favors CE over BAE and other radiologic studies. A cost-effectiveness analysis for management of bleeding favors BAE over all other therapeutic modalities. CE is patient friendly, guides BAE approach in positive studies, and predicts a low re-bleeding rate in negative studies. BAE is invasive and requires significant resources and time. It may be occasionally needed to extract a retained capsule endoscope at the SB stricture. Finally, BAE should be considered in patients with a negative CE who continue to have unexplained IDA and OGIB.
CE enables visualization of the entire SB but lacks the potential for therapeutic intervention. Other limitations include inability to provide precise location of a lesion, false-positive results, potential for erratic passage resulting in missed lesions and limited battery life causing incomplete studies.
CE is a very safe procedure with few reported adverse events. There have been concerns about the theoretical interaction of CE with cardiac defibrillators, although no adverse events have been reported in the literature[41,42]. Inability to swallow the capsule, battery failure before capsule reaches the cecum, and capsule retention are some of the important problems associated with CE in elderly as well as in younger patients[43]. In patients who are unable to swallow the capsule, it can be successfully placed in the duodenum by using a capsule delivery device. In patients with suspected SB stricture, a new capsule (AGILE Patency system by Given Imaging) is available, which can be given before SB capsule to evaluate for SB patency[44]. The true remaining contraindications to CE are SB obstruction/pseudo-obstruction and pregnancy[45].
The use of GI endoscopy in geriatric patients is increasing as a larger proportion of the population is reaching an advanced age. IDA is a major problem in elderly and requires further evaluation and management. SB CE plays an important role in the evaluation of IDA in all age groups, especially in the elderly with a very high DY. SB angioectasia, ulceration, tumor, CD and active bleeding are the common findings seen on CE in elderly with IDA. BAE is used to treat the lesions seen on CE. CE has some limitations mainly lack of therapeutic capability, inability to provide precise location of the lesion and false positive results. Overall CE is a very safe and effective procedure for the evaluation of IDA in elderly.
P- Reviewers: Ersoy O, Iakovidis DK S- Editor: Zhai HH L- Editor: A E- Editor: Zhang DN
| 1. | Herbert V. Megaloblastic anemia as a problem in world health. Am J Clin Nutr. 1968;21:1115-1120. [PubMed] [Cited in This Article: ] |
| 2. | Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973-976. [PubMed] [Cited in This Article: ] |
| 3. | Zakai NA, Katz R, Hirsch C, Shlipak MG, Chaves PH, Newman AB, Cushman M. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: the Cardiovascular Health Study. Arch Intern Med. 2005;165:2214-2220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 319] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 4. | Penninx BW, Guralnik JM, Onder G, Ferrucci L, Wallace RB, Pahor M. Anemia and decline in physical performance among older persons. Am J Med. 2003;115:104-110. [PubMed] [Cited in This Article: ] |
| 5. | Dharmarajan TS, Pais W, Norkus EP. Does anemia matter? Anemia, morbidity, and mortality in older adults: need for greater recognition. Geriatrics. 2005;60:22-7, 29. [PubMed] [Cited in This Article: ] |
| 6. | Melmed GY, Lo SK. Capsule endoscopy: practical applications. Clin Gastroenterol Hepatol. 2005;3:411-422. [PubMed] [Cited in This Article: ] |
| 7. | American Gastroenterological Association medical position statement: evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology. 2000;118:197-201. [PubMed] [Cited in This Article: ] |
| 8. | Fisher L, Lee Krinsky M, Anderson MA, Appalaneni V, Banerjee S, Ben-Menachem T, Cash BD, Decker GA, Fanelli RD, Friis C. The role of endoscopy in the management of obscure GI bleeding. Gastrointest Endosc. 2010;72:471-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1994] [Cited by in F6Publishing: 1333] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 10. | Iddan GJ, Swain CP. History and development of capsule endoscopy. Gastrointest Endosc Clin N Am. 2004;14:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Fritscher-Ravens A, Swain CP. The wireless capsule: new light in the darkness. Dig Dis. 2002;20:127-133. [PubMed] [Cited in This Article: ] |
| 12. | Tang SJ, Haber GB. Capsule endoscopy in obscure gastrointestinal bleeding. Gastrointest Endosc Clin N Am. 2004;14:87-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Raju GS, Gerson L, Das A, Lewis B. American Gastroenterological Association (AGA) Institute medical position statement on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1694-1696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Fireman Z, Friedman S. Diagnostic yield of capsule endoscopy in obscure gastrointestinal bleeding. Digestion. 2004;70:201-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Leighton JA, Triester SL, Sharma VK. Capsule endoscopy: a meta-analysis for use with obscure gastrointestinal bleeding and Crohn’s disease. Gastrointest Endosc Clin N Am. 2006;16:229-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Mishkin DS, Chuttani R, Croffie J, Disario J, Liu J, Shah R, Somogyi L, Tierney W, Song LM, Petersen BT. ASGE Technology Status Evaluation Report: wireless capsule endoscopy. Gastrointest Endosc. 2006;63:539-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 17. | Pennazio M, Santucci R, Rondonotti E, Abbiati C, Beccari G, Rossini FP, De Franchis R. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology. 2004;126:643-653. [PubMed] [Cited in This Article: ] |
| 18. | Richter JM, Christensen MR, Colditz GA, Nishioka NS. Angiodysplasia. Natural history and efficacy of therapeutic interventions. Dig Dis Sci. 1989;34:1542-1546. [PubMed] [Cited in This Article: ] |
| 19. | Foutch PG. Angiodysplasia of the gastrointestinal tract. Am J Gastroenterol. 1993;88:807-818. [PubMed] [Cited in This Article: ] |
| 20. | Holleran G, Hall B, Hussey M, McNamara D. Small bowel angiodysplasia and novel disease associations: a cohort study. Scand J Gastroenterol. 2013;48:433-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Okazaki H, Fujiwara Y, Sugimori S, Nagami Y, Kameda N, Machida H, Yamagami H, Tanigawa T, Shiba M, Watanabe K. Prevalence of mid-gastrointestinal bleeding in patients with acute overt gastrointestinal bleeding: multi-center experience with 1,044 consecutive patients. J Gastroenterol. 2009;44:550-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Chutkan R, Toubia N. Effect of nonsteroidal anti-inflammatory drugs on the gastrointestinal tract: diagnosis by wireless capsule endoscopy. Gastrointest Endosc Clin N Am. 2004;14:67-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Rondonotti E, Pennazio M, Toth E, Menchen P, Riccioni ME, De Palma GD, Scotto F, De Looze D, Pachofsky T, Tacheci I. Small-bowel neoplasms in patients undergoing video capsule endoscopy: a multicenter European study. Endoscopy. 2008;40:488-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Eliakim R, Adler SN. Capsule video endoscopy in Crohn’s disease-the European experience. Gastrointest Endosc Clin N Am. 2004;14:129-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Sidhu R, Sanders DS, Kapur K, Leeds JS, McAlindon ME. Factors predicting the diagnostic yield and intervention in obscure gastrointestinal bleeding investigated using capsule endoscopy. J Gastrointestin Liver Dis. 2009;18:273-278. [PubMed] [Cited in This Article: ] |
| 26. | Sidhu R, McAlindon ME, Drew K, Hardcastle S, Cameron IC, Sanders DS. Evaluating the role of small-bowel endoscopy in clinical practice: the largest single-centre experience. Eur J Gastroenterol Hepatol. 2012;24:513-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Muhammad A, Pitchumoni CS. Evaluation of iron deficiency anemia in older adults: the role of wireless capsule endoscopy. J Clin Gastroenterol. 2009;43:627-631. [PubMed] [Cited in This Article: ] |
| 28. | Muhammad A, Pitchumoni CS. Newly detected celiac disease by wireless capsule endoscopy in older adults with iron deficiency anemia. J Clin Gastroenterol. 2008;42:980-983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Koulaouzidis A, Rondonotti E, Giannakou A, Plevris JN. Diagnostic yield of small-bowel capsule endoscopy in patients with iron-deficiency anemia: a systematic review. Gastrointest Endosc. 2012;76:983-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Appleyard M, Fireman Z, Glukhovsky A, Jacob H, Shreiver R, Kadirkamanathan S, Lavy A, Lewkowicz S, Scapa E, Shofti R. A randomized trial comparing wireless capsule endoscopy with push enteroscopy for the detection of small-bowel lesions. Gastroenterology. 2000;119:1431-1438. [PubMed] [Cited in This Article: ] |
| 31. | Gerson L, Kamal A. Cost-effectiveness analysis of management strategies for obscure GI bleeding. Gastrointest Endosc. 2008;68:920-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Domagk D, Mensink P, Aktas H, Lenz P, Meister T, Luegering A, Ullerich H, Aabakken L, Heinecke A, Domschke W. Single- vs. double-balloon enteroscopy in small-bowel diagnostics: a randomized multicenter trial. Endoscopy. 2011;43:472-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 33. | Takano N, Yamada A, Watabe H, Togo G, Yamaji Y, Yoshida H, Kawabe T, Omata M, Koike K. Single-balloon versus double-balloon endoscopy for achieving total enteroscopy: a randomized, controlled trial. Gastrointest Endosc. 2011;73:734-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 34. | Efthymiou M, Desmond PV, Brown G, La Nauze R, Kaffes A, Chua TJ, Taylor AC. SINGLE-01: a randomized, controlled trial comparing the efficacy and depth of insertion of single- and double-balloon enteroscopy by using a novel method to determine insertion depth. Gastrointest Endosc. 2012;76:972-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Muravchick S. The elderly outpatient: current anesthetic implications. Curr Opin Anaesthesiol. 2002;15:621-625. [PubMed] [Cited in This Article: ] |
| 36. | Gerson LB, Tokar J, Chiorean M, Lo S, Decker GA, Cave D, Bouhaidar D, Mishkin D, Dye C, Haluszka O. Complications associated with double balloon enteroscopy at nine US centers. Clin Gastroenterol Hepatol. 2009;7:1177-182, 1177-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 37. | Möschler O, May A, Müller MK, Ell C. Complications in and performance of double-balloon enteroscopy (DBE): results from a large prospective DBE database in Germany. Endoscopy. 2011;43:484-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 38. | Hegde SR, Iffrig K, Li T, Downey S, Heller SJ, Tokar JL, Haluszka O. Double-balloon enteroscopy in the elderly: safety, findings, and diagnostic and therapeutic success. Gastrointest Endosc. 2010;71:983-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Gómez V, Cheesman AR, Heckman MG, Rawal B, Stark ME, Lukens FJ. Safety of capsule endoscopy in the octogenarian as compared with younger patients. Gastrointest Endosc. 2013;78:744-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Teshima CW, Kuipers EJ, van Zanten SV, Mensink PB. Double balloon enteroscopy and capsule endoscopy for obscure gastrointestinal bleeding: an updated meta-analysis. J Gastroenterol Hepatol. 2011;26:796-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 41. | Leighton JA, Srivathsan K, Carey EJ, Sharma VK, Heigh RI, Post JK, Erickson PJ, Robinson SR, Bazzell JL, Fleischer DE. Safety of wireless capsule endoscopy in patients with implantable cardiac defibrillators. Am J Gastroenterol. 2005;100:1728-1731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Payeras G, Piqueras J, Moreno VJ, Cabrera A, Menéndez D, Jiménez R. Effects of capsule endoscopy on cardiac pacemakers. Endoscopy. 2005;37:1181-1185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Orlando G, Luppino IM, Lerose MA, Gervasi R, Amato B, Silecchia G, Puzziello A. Feasibility of capsule endoscopy in elderly patients with obscure gastrointestinal bleeding. An up-to-date report. BMC Surg. 2012;12 Suppl 1:S30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Signorelli C, Rondonotti E, Villa F, Abbiati C, Beccari G, Avesani EC, Vecchi M, de Franchis R. Use of the Given Patency System for the screening of patients at high risk for capsule retention. Dig Liver Dis. 2006;38:326-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Storch I, Barkin JS. Contraindications to capsule endoscopy: do any still exist? Gastrointest Endosc Clin N Am. 2006;16:329-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Holleran GE, Barry SA, Thornton OJ, Dobson MJ, McNamara DA. The use of small bowel capsule endoscopy in iron deficiency anaemia: low impact on outcome in the medium term despite high diagnostic yield. Eur J Gastroenterol Hepatol. 2013;25:327-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Tong J, Svarta S, Ou G, Kwok R, Law J, Enns R. Diagnostic yield of capsule endoscopy in the setting of iron deficiency anemia without evidence of gastrointestinal bleeding. Can J Gastroenterol. 2012;26:687-690. [PubMed] [Cited in This Article: ] |
| 48. | Koulaouzidis A, Yung DE, Lam JH, Smirnidis A, Douglas S, Plevris JN. The use of small-bowel capsule endoscopy in iron-deficiency anemia alone; be aware of the young anemic patient. Scand J Gastroenterol. 2012;47:1094-1100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Yamada A, Watabe H, Yamaji Y, Yoshida H, Omata M, Koike K. Incidence of small intestinal lesions in patients with iron deficiency anemia. Hepatogastroenterology. 2011;58:1240-1243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Efthymiou M, Allen PB, Jayasekera C, Taylor PV, Taylor AC. Value of fecal occult blood test as a screening test before capsule endoscopy. Eur J Gastroenterol Hepatol. 2011;23:690-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Milano A, Balatsinou C, Filippone A, Caldarella MP, Laterza F, Lapenna D, Pierdomenico SD, Pace F, Cuccurullo F, Neri M. A prospective evaluation of iron deficiency anemia in the GI endoscopy setting: role of standard endoscopy, videocapsule endoscopy, and CT-enteroclysis. Gastrointest Endosc. 2011;73:1002-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Goenka MK, Majumder S, Kumar S, Sethy PK, Goenka U. Single center experience of capsule endoscopy in patients with obscure gastrointestinal bleeding. World J Gastroenterol. 2011;17:774-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 57] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Katsinelos P, Chatzimavroudis G, Terzoudis S, Patsis I, Fasoulas K, Katsinelos T, Kokonis G, Zavos C, Vasiliadis T, Kountouras J. Diagnostic yield and clinical impact of capsule endoscopy in obscure gastrointestinal bleeding during routine clinical practice: a single-center experience. Med Princ Pract. 2011;20:60-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Riccioni ME, Urgesi R, Spada C, Cianci R, Pelecca G, Bizzotto A, Costamagna G. Unexplained iron deficiency anaemia: Is it worthwhile to perform capsule endoscopy? Dig Liver Dis. 2010;42:560-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | van Turenhout ST, Jacobs MA, van Weyenberg SJ, Herdes E, Stam F, Mulder CJ, Bouma G. Diagnostic yield of capsule endoscopy in a tertiary hospital in patients with obscure gastrointestinal bleeding. J Gastrointestin Liver Dis. 2010;19:141-145. [PubMed] [Cited in This Article: ] |
| 56. | Laine L, Sahota A, Shah A. Does capsule endoscopy improve outcomes in obscure gastrointestinal bleeding? Randomized trial versus dedicated small bowel radiography. Gastroenterology. 2010;138:1673-1680.e1; quiz e11-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 57. | Sheibani S, Levesque BG, Friedland S, Roost J, Gerson LB. Long-term impact of capsule endoscopy in patients referred for iron-deficiency anemia. Dig Dis Sci. 2010;55:703-708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Kim S, Kedia PS, Jaffe DL, Ahmad NA. Impact of capsule endoscopy findings on patient outcomes. Dig Dis Sci. 2009;54:2441-2448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Chami G, Raza M, Bernstein CN. Usefulness and impact on management of positive and negative capsule endoscopy. Can J Gastroenterol. 2007;21:577-581. [PubMed] [Cited in This Article: ] |
| 60. | Carey EJ, Leighton JA, Heigh RI, Shiff AD, Sharma VK, Post JK, Fleischer DE. A single-center experience of 260 consecutive patients undergoing capsule endoscopy for obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102:89-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 61. | Apostolopoulos P, Liatsos C, Gralnek IM, Giannakoulopoulou E, Alexandrakis G, Kalantzis C, Gabriel P, Kalantzis N. The role of wireless capsule endoscopy in investigating unexplained iron deficiency anemia after negative endoscopic evaluation of the upper and lower gastrointestinal tract. Endoscopy. 2006;38:1127-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 62. | Estévez E, González-Conde B, Vázquez-Iglesias JL, de Los Angeles Vázquez-Millán M, Pértega S, Alonso PA, Clofent J, Santos E, Ulla JL, Sánchez E. Diagnostic yield and clinical outcomes after capsule endoscopy in 100 consecutive patients with obscure gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2006;18:881-888. [PubMed] [Cited in This Article: ] |
| 63. | Van Tuyl SA, Van Noorden JT, Kuipers EJ, Stolk MF. Results of videocapsule endoscopy in 250 patients with suspected small bowel pathology. Dig Dis Sci. 2006;51:900-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Qvigstad G, Hatlen-Rebhan P, Brenna E, Waldum HL. Capsule endoscopy in clinical routine in patients with suspected disease of the small intestine: a 2-year prospective study. Scand J Gastroenterol. 2006;41:614-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Kalantzis N, Papanikolaou IS, Giannakoulopoulou E, Alogari A, Kalantzis C, Papacharalampous X, Gabriel P, Alexandrakis G, Apostolopoulos P. Capsule endoscopy; the cumulative experience from its use in 193 patients with suspected small bowel disease. Hepatogastroenterology. 2005;52:414-419. [PubMed] [Cited in This Article: ] |
| 66. | De Leusse A, Landi B, Edery J, Burtin P, Lecomte T, Seksik P, Bloch F, Jian R, Cellier C. Video capsule endoscopy for investigation of obscure gastrointestinal bleeding: feasibility, results, and interobserver agreement. Endoscopy. 2005;37:617-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Ben Soussan E, Antonietti M, Hervé S, Savoye G, Ramirez S, Lecleire S, Ducrotté P, Lerebours E. Diagnostic yield and therapeutic implications of capsule endoscopy in obscure gastrointestinal bleeding. Gastroenterol Clin Biol. 2004;28:1068-1073. [PubMed] [Cited in This Article: ] |
| 68. | Enns R, Go K, Chang H, Pluta K. Capsule endoscopy: a single-centre experience with the first 226 capsules. Can J Gastroenterol. 2004;18:555-558. [PubMed] [Cited in This Article: ] |
| 69. | Fireman Z, Eliakim R, Adler S, Scapa E. Capsule endoscopy in real life: a four-centre experience of 160 consecutive patients in Israel. Eur J Gastroenterol Hepatol. 2004;16:927-931. [PubMed] [Cited in This Article: ] |