Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Jun 14, 2014; 20(22): 7019-7026
Published online Jun 14, 2014. doi: 10.3748/wjg.v20.i22.7019
Published online Jun 14, 2014. doi: 10.3748/wjg.v20.i22.7019
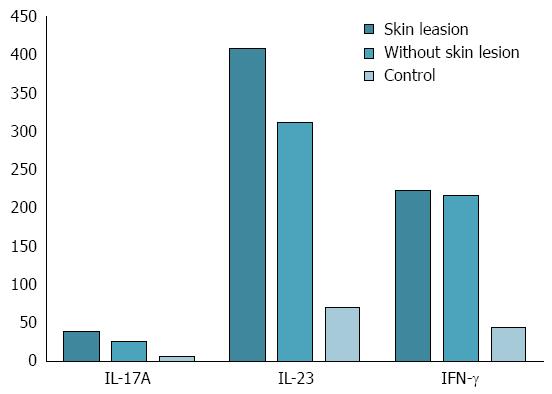
Figure 2 Interleukin 17A, interleukin 23 and interferon gamma serum concentration in controls and Crohn’s disease patients with and without skin adverse side effects of anti-tumor necrosis factor-α therapy.
Interleukin 17A (IL-17A): With skin lesions vs without skin lesions: P = 0.000044; with skin lesions vs control: P = 0.000005; without skin lesions vs control: P = 0.000037; IL-23: With skin lesions vs without skin lesions: P = 0.005557; with skin lesions vs control: P = 0.000005; without skin lesions vs control: P = 0.000037; interferon gamma (IFN-γ): With skin lesions vs without skin lesions: P = 0.966233; with skin lesions vs control: P = 0.000005; without skin lesions vs control: P = 0.000037.
- Citation: Włodarczyk M, Sobolewska A, Wójcik B, Loga K, Fichna J, Wiśniewska-Jarosińska M. Correlations between skin lesions induced by anti-tumor necrosis factor-α and selected cytokines in Crohn's disease patients. World J Gastroenterol 2014; 20(22): 7019-7026
- URL: https://www.wjgnet.com/1007-9327/full/v20/i22/7019.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i22.7019