Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. May 21, 2014; 20(19): 5773-5793
Published online May 21, 2014. doi: 10.3748/wjg.v20.i19.5773
Published online May 21, 2014. doi: 10.3748/wjg.v20.i19.5773
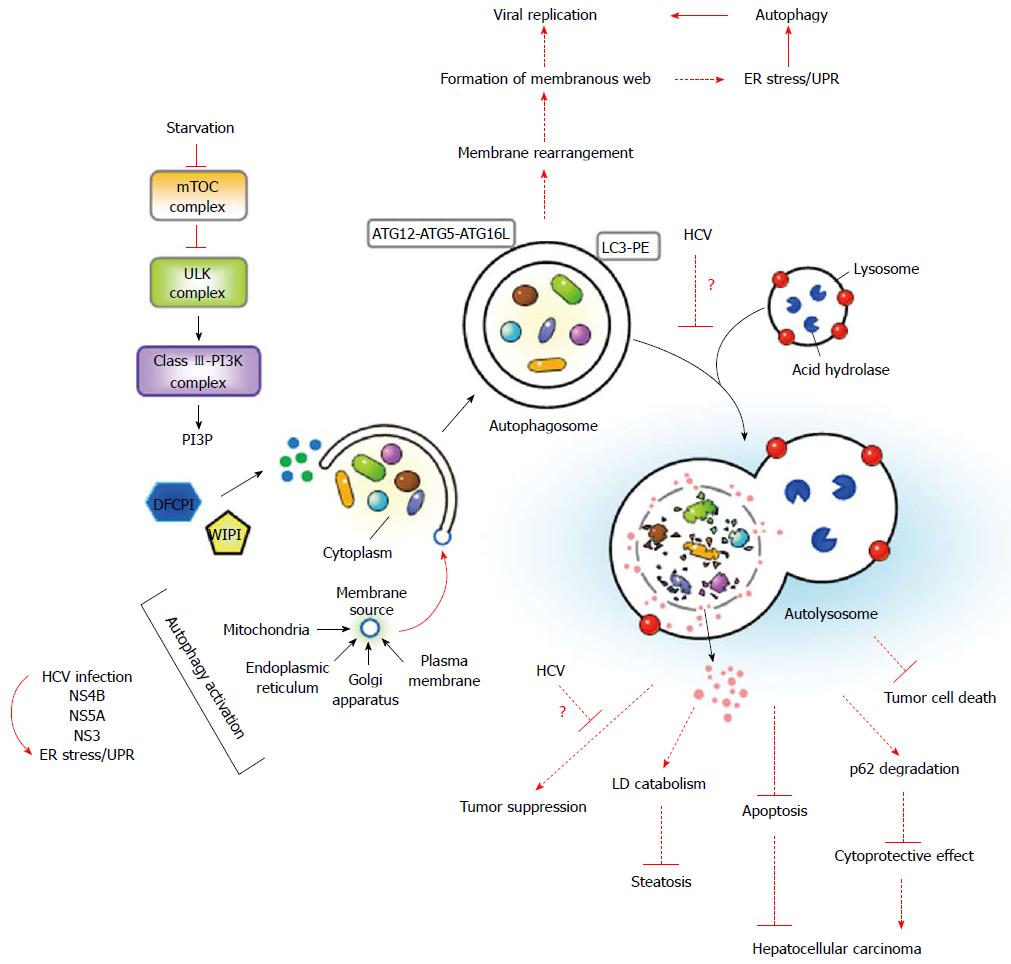
Figure 4 Sequential and coordinated events of autophagy and their impact on viral replication and hepatitis C virus-associated liver diseases.
When cells undergo nutrient starvation, the activity of the mammalian target of rapamycin (mTOR) complex is inhibited, resulting in dephosphorylation, activation, and translocation of the unc-51 like-kinase (ULK) complex to the ER, where the ULK complex activates the class III phosphatidylinositol-3-OH kinase (class III-PI3K) complex to generate PtdIn(3)P (PI3P). PI3P, in turn, recruits double-FYVE-containing protein 1 (DFCP1) and WD-repeat domain PI3P-interacting (WIPI) protein into the isolation membrane, which may originate from the ER, mitochondria, plasma membrane, or Golgi apparatus. Two ubiquitin-like conjugation systems, the ATG12-ATG5-ATG16L and LC3-PE conjugation cascades, coordinate the elongation and enclosure of the autophagosome. Finally, the autophagosome fuses with a lysosome to degrade the sequestrated cytoplasmic components. Hepatitis C virus (HCV) infection, which activates ER stress/the unfolded protein response (UPR), as marked by a curved arrow, and ectopic expression of NS4B, NS5A, and NS3 are known to activate autophagy. The dashed lines indicate the potential implications of autophagy in HCV replication, HCV-related steatosis, and hepatocellular carcinoma. Activated autophagy may contribute to the rearrangement of the membrane as a resource of the membranous web for viral RNA replication. It is surmised that the HCV-induced blockade in autolysosome formation, as shown by a “?” during fusion of the autophagosome with a lysosome, may disrupt the effect of autophagy on LD and p62 degradation, thus contributing to the development of steatosis and hepatocellular carcinoma. On the other hand, interference with the effects of autophagy on tumor suppression by HCV, as indicted by a “?”, or the inhibitory effects of autophagy on the apoptotic signaling and killing of tumor cells in HCV-infected patients may also enhance the progression to hepatocellular carcinoma.
- Citation: Ke PY, Chen SSL. Autophagy in hepatitis C virus-host interactions: Potential roles and therapeutic targets for liver-associated diseases. World J Gastroenterol 2014; 20(19): 5773-5793
- URL: https://www.wjgnet.com/1007-9327/full/v20/i19/5773.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i19.5773