Published online May 7, 2014. doi: 10.3748/wjg.v20.i17.4963
Revised: December 25, 2013
Accepted: March 5, 2014
Published online: May 7, 2014
Processing time: 193 Days and 20 Hours
AIM: To investigate whether naofen is involved in tumor necrosis factor (TNF)-α-mediated apoptosis of hepatocytes induced by lipopolysaccharide (LPS).
METHODS: In vivo, rats were treated with LPS or anti-TNF-α antibody, whereas in vitro, primary hepatocytes and Kupffer cells (KCs) were separately isolated from rat livers using collagenase perfusion, and primary hepatocytes were cultured in medium containing LPS or TNF-α, or in conditioned medium from LPS-treated KCs (KC-CM)/KC-CM + anti-TNF-α antibody. Naofen and TNF-α mRNA expression was examined by real-time reverse transcription-polymerase chain reaction. Immunoblotting was used to measure protein expression. Hepatocyte apoptosis was determined by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay.
RESULTS: LPS significantly induced both naofen expression and caspase-3 activity in the rat liver, which coincided with an increase in the number of TUNEL-positive hepatocytes. The increase of TNF-α expression induced by LPS was preceded by increases in naofen and caspase-3 activity. Elevation of naofen expression and caspase-3 activity was abrogated by pretreatment with anti-TNF-α antibody. In KCs, LPS caused an increase in TNF-α that was almost consistent with that in the liver of LPS-treated rats. In hepatocytes, neither LPS nor TNF-α alone affected either naofen expression or caspase-3 activation. The incubation of hepatocytes with KC-CM significantly enhanced both naofen expression and caspase-3 activity. Moreover, the effects of the KC-CM-induced increase in naofen expression and caspase-3 activity were blocked by anti-TNF-α antibody.
CONCLUSION: TNF-α released from KCs treated with LPS may induce hepatic naofen expression, which then stimulates hepatocellular apoptosis through activation of caspase-3.
Core tip: Naofen, a WD40-repeat protein, is increased in the liver but not in the kidneys, thymus or spleen of rats injected with lipopolysaccharide (LPS). Increased naofen expression is blocked by pretreatment with anti-tumor necrosis factor (TNF)-α antibody. TNF-α has no effect on naofen expression or caspase-3 activation in primary hepatocytes, but conditioned medium from LPS-treated Kupffer cells (KC-CM) significantly enhances both. KC-CM-induced increase in naofen expression and caspase-3 activity is blocked by anti-TNF-α antibody. LPS in the liver may enhance release of TNF-α from KCs, and induce hepatocyte apoptosis, for which naofen promotes caspase-3 activity through the mitochondrial pathway.
- Citation: Fan JH, Feng GG, Huang L, Tang GD, Jiang HX, Xu J. Naofen promotes TNF-α-mediated apoptosis of hepatocytes by activating caspase-3 in lipopolysaccharide-treated rats. World J Gastroenterol 2014; 20(17): 4963-4971
- URL: https://www.wjgnet.com/1007-9327/full/v20/i17/4963.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i17.4963
Lipopolysaccharide (LPS) is a major structural component of the outer membrane of Gram-negative bacteria[1]. Under normal conditions, a small amount of LPS, mainly from the intestine, can periodically be taken up into the liver through the portal vein and then scavenged by Kupffer cells (KCs), the resident macrophages in the liver[2]. The liver functions as the first barrier to LPS entering the circulation and as a detoxification organ, therefore, it is deeply affected by endotoxemia. However, in patients with severe trauma, burns, intestinal ischemia and liver diseases, LPS can spill over into the systemic circulation because of the increased permeability of the intestinal wall and/or the decreased phagocytic ability of liver KCs[3-5]. Under septic conditions, LPS-induced hepatocyte death may have a role in liver dysfunction, possibly associated with apoptosis of hepatocytes[5-7]. It is clear that LPS does not directly have pathogenetic roles, but rather the effects are mainly dependent on the production and release of potent inflammatory mediators, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, and IL-10[8,9]. These mediators, especially TNF-α, can induce apoptotic liver injury[10] and the infiltration of inflammatory cells. The latter, in turn, can further exacerbate liver injury, which continues the vicious circle of infiltration/liver injury[3,6-8]. A number of inflammatory liver diseases in humans, including viral hepatitis, alcoholic liver disease, immune- or drug-induced liver injury and ischemia/reperfusion liver failure, have been shown to be dependent on TNF-α production[5,11,12]. Therefore, to control liver damage under such pathological conditions, it may be important to understand the functions of TNF-α in liver injuries.
Hepatocyte apoptosis, as a general feature, is the most important event in the molecular mechanisms of hepatic failure, because apoptosis is the first cellular response of the liver to a wide range of toxic substances (including LPS), and necrosis in hepatic tissues is often found to follow the appearance of apoptosis[13-15]. It has been well documented that the caspase cascade involved in apoptosis includes both initiator and effector caspases[13-15]. Two main initiator caspases, caspase-8 and caspase-9, mediate distinct sets of death signals. Caspase-8 is activated by death signals that bind to death receptors on the cell surface. In contrast, caspase-9 is activated by cytochrome c released from mitochondria. Proapoptotic signals activate an initiator caspase that, in turn, activates effector caspases, for example, caspase-3. Sequential activation of caspases results in cleavage of substrate proteins and breakdown of DNA molecules, leading to apoptosis. So far, although many studies of hepatocyte apoptosis have been conducted, the precise molecular mechanisms remain incompletely defined. Therefore, the identification of signal pathways in LPS-mediated hepatocyte apoptosis would contribute to understanding the pathophysiological roles of apoptosis in liver diseases.
Recently, naofen was found as an intracellular protein reactive to anti-verotoxin II antibody and classified in the aspartate-tryptophan (WD) 40-repeat protein family[16]. In deoxycorticosterone-induced renal hypertension in rats, naofen is increased in vascular endothelial cells and suppresses nitric oxide synthesis[16]. Naofen also induces apoptosis in streptozotocin-induced diabetic rat kidney[17] and mediates spontaneous and TNF-α induced apoptosis in human embryonic kidney (HEK) 293 cells[18]. Furthermore, naofen was increased in hepatocytes, causing apoptosis in LPS-treated rat liver[19]. Thus, it was hypothesized that naofen may be involved in TNF-α-induced apoptosis of hepatocytes. The present study was undertaken to examine whether naofen participates in the TNF-α-mediated apoptosis of hepatocytes in LPS-treated rats. Moreover, the correlating mechanisms were evaluated, utilizing primary cultures of KCs and hepatocytes.
Male Sprague-Dawley rats (weighing 200-250 g; SLC Inc., Guangxi, China) were maintained in climate-controlled rooms under a 12-h light-dark cycle. All experiments were conducted in accordance with the Institutional Guidelines of Guangxi Medical University for the care and use of laboratory animals.
Rats were injected with LPS (500 μg/kg; Sigma, St. Louis, MO, United States) via the femoral vein under ether anesthesia, and saline was used as a control as previously reported[19]. A second set of experiments was performed to determine the influence of anti-TNF-α on the expression of naofen in response to LPS. Rats received femoral vein injection of nonspecific IgG (2 mg/kg; Biosensis, Thebarton, SA, Australia) + LPS (500 μg/kg; Sigma), anti-TNF-α (2 mg/kg; R and D Systems, Minneapolis, MN, United States) + LPS (500 μg/kg), saline + IgG (2 mg/kg), or saline + anti-TNF-α (2 mg/kg). The anti-TNF-α and IgG were administered 24 h before LPS. Ten rats were used for each time point. At 1, 3, 6, 9 and 12 h after injection, animals were anesthetized with pentobarbital sodium (50 mg/kg intraperitoneally), and blood samples were collected from the inferior vena cava. The livers were removed, immediately frozen, and stored in liquid nitrogen for RNA and protein extraction.
Hepatocytes and KCs were separately prepared from the livers of Sprague-Dawley rats using collagenase perfusion[20,21]. Hepatocytes were cultivated in Williams’ E medium containing 10% calf serum, 2 mmol L-glutamate and antibiotics (100 U/mL penicillin G and 100 μg/mL streptomycin sulfate). KCs were cultured with RPMI 1640 medium containing 10% calf serum and antibiotics. Hepatocytes (1 × 106) and 5 × 105 KCs per well were plated on a 6-cm plate and incubated at 37 °C under 5% CO2 and 95% O2 for 6 and 1 h, respectively. The culture medium was then changed to remove nonviable and unattached cells. The viability of cells tested by trypan blue dye exclusion ranged between 87% and 95%. The purity of hepatocytes examined by light microscopy and of KCs identified by phagocytosis of latex beads (polystyrene beads, mean particle size 1.1 μm; Sigma) ranged between 85% and 95%. Duplicate cultures were prepared for each treatment, and independent experiments were performed at least four times.
After 24 h of culture, KCs were incubated in medium containing 100 ng/mL LPS for 1-12 h, and TNF-α expression was measured. In some experiments, KCs were treated with LPS for 6 h, and the culture medium, as Kupffer cell-conditioned medium (KC-CM), was collected and centrifuged at 15000 g at 4 °C for 10 min to remove cell debris. To confirm the effects of TNF-α, an antibody against TNF-α (500 ng/mL) was added to KC-CM (6 h) and incubated at 37 °C for 1 h (6 h KC-CM + anti-TNF-α). Hepatocytes were incubated respectively with LPS (100 ng/mL), TNF-α (10 ng/mL), IgG (500 ng/mL), 6 h KC-CM or 6 h KC-CM + anti-TNF-α for 12 h, and the expression of naofen, TNF-α and caspase-3 activity was analyzed.
Total RNA (1 μg) was extracted from livers or primary cells using TRIzol reagent (Invitrogen, Carlsbad, CA, United States) and reversely transcribed using a ReverTra Ace quantitative PCR (qPCR) RT kit (Toyobo, Osaka, Japan) according to the manufacturer’s instructions. Target mRNA expression was quantified using qPCR as described previously[19]. The primers and probe for naofen (forward primer 5’-CGATTTCTGCATTTTGGCCACAA-3’, reverse primer 5’-TCCAAGGGTGTGCCAATAGAATT-3 and TaqMan MGB probe 5’-CAAACTGAGGGTGATTTT-3’) and TaqMan Gene Expression Assays for naofen (ID: Rn01769571_m1), TNF-α (ID: Rn99999017_m1), GAPDH (ID: Rn99999916_s1) and β-actin (ID: Rn00667869_m1) were purchased from Applied Biosystems (Foster City, CA, United States).
Protein samples (30-50 μg) were prepared from livers and cells and separated by SDS-PAGE, followed by transfer to PVDF membranes (Millipore, Billerica, MA, United States) as reported previously[19]. Blots were incubated with an anti-naofen antibody (anti-NF, 1:500), which was designed and produced by Medical & Biological Laboratories (Nagoya, Japan) or antibodies (1:1000, respectively) against TNF-α and GAPDH (Cell Signaling Technology, Danvers, MA, United States), followed by incubation with a peroxidase-conjugated goat IgG (1:5000; Sigma). Proteins were visualized using ECL Plus Western blotting Reagent (GE Healthcare, Chalfont St Giles, Bucks, United Kingdom). Changes in target protein levels were measured quantitatively using Image J (free software made by NIH initiative).
Caspase-3 activation was determined using a Caspase Fluorometric assay kit (Medical and Biological Laboratories) as previously reported[19]. Free AFC cleaved by caspase-3 from the substrate, DEVD (Asp-Glu-Val-Asp)-AFC (7-amino-4-trifluoromethyl coumarin), was quantified by Fluoroskan Ascent FL (Labsystems, Helsinki, Finland) with excitation/emission (Ex/Em) = 400/505 nm.
Livers were fixed with 4% paraformaldehyde in PBS and embedded in paraffin. Serial 5-μm sections were made for terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay, a method for detecting DNA fragmentation in apoptosis, using an ApopTag Plus peroxidase in situ apoptosis detection kit (Millipore) according to the manufacturer’s instructions[22]. For each sample, five high-power fields (× 200) were randomly selected, each containing an average of 400 cells, and the number of apoptotic cells was counted for each field. Apoptosis index (AI) (%) was calculated as number of positive cells/number of total cells × 100%.
Results are expressed as mean ± SE (n = 10), unless otherwise indicated. Statistical analyses were performed using Kruskal-Wallis one-way analysis of variance. P < 0.05 was considered significant.
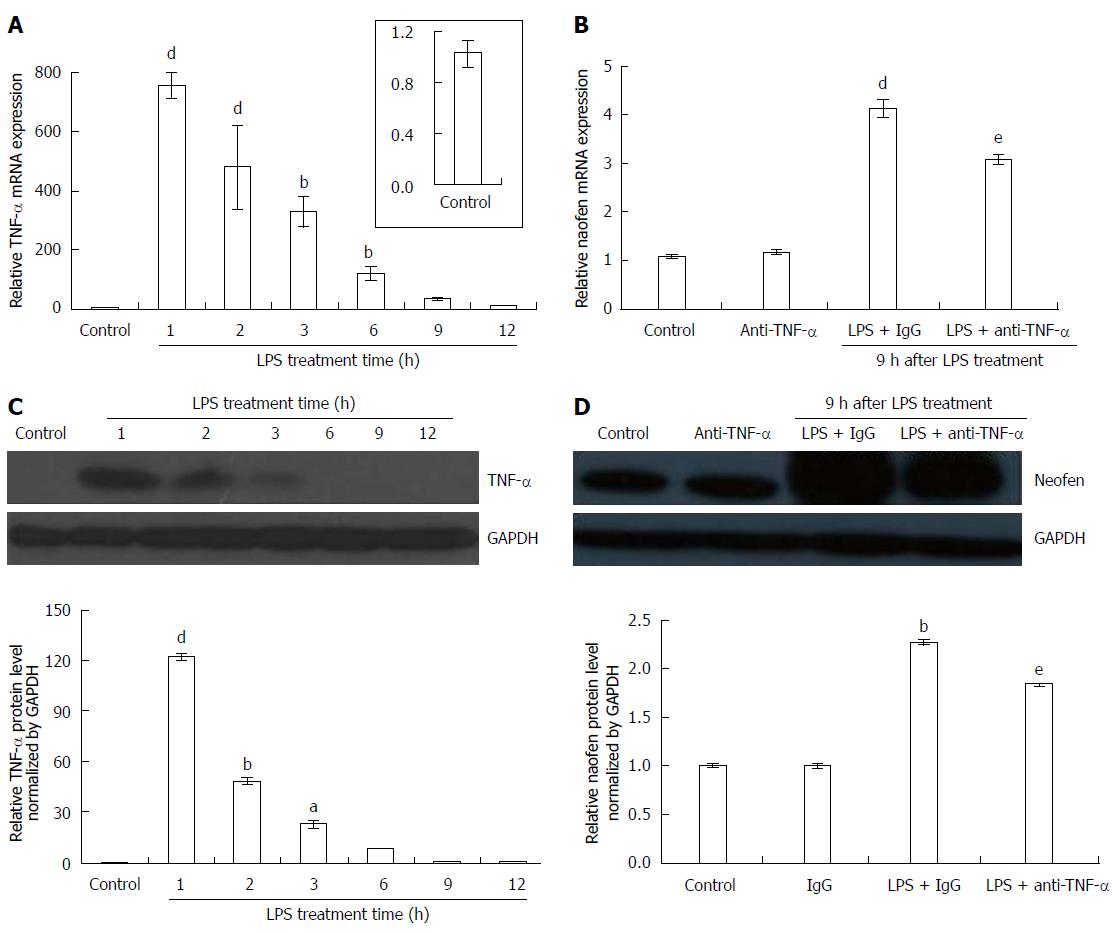
Changes in the time course of TNF-α expression were investigated in the livers of rats injected with 500 μg/kg LPS + 2 mg/kg IgG for 1-12 h. TNF-α mRNA rapidly increased by the greatest amount within 1 h after injection, and then gradually decreased (Figure 1A). In the immunoblotting assay with anti-TNF-α (Figure 1C), compared to the control saline + IgG in which TNF-α was almost undetectable, LPS resulted in the strongest signal intensity for TNF-α protein after 1 h injection, then diminished, and recovered to an undetectable level within 12 h.
As previously reported[19], the expression of naofen was increased from 5 μg/kg LPS and peaked at 500 μg/kg. In addition, naofen mRNA increased from 3 h, peaked at 9 h, and then diminished. Thus, changes in naofen expression were investigated using 500 μg/kg LPS + 2 mg/kg IgG. In contrast to the control saline + IgG, naofen expression was obviously increased at 9 h (Figure 1B). Immunoreactivity for naofen also appeared to have a similar pattern with its mRNA expression (Figure 1D).
Gene expression and protein level for naofen were found to be significantly reduced in rats treated with 2 mg/kg anti-TNF-α + LPS compared to LPS + IgG (Figure 1B and D). The expression of TNF-α and naofen were not significantly different between saline and saline + IgG (data not shown).
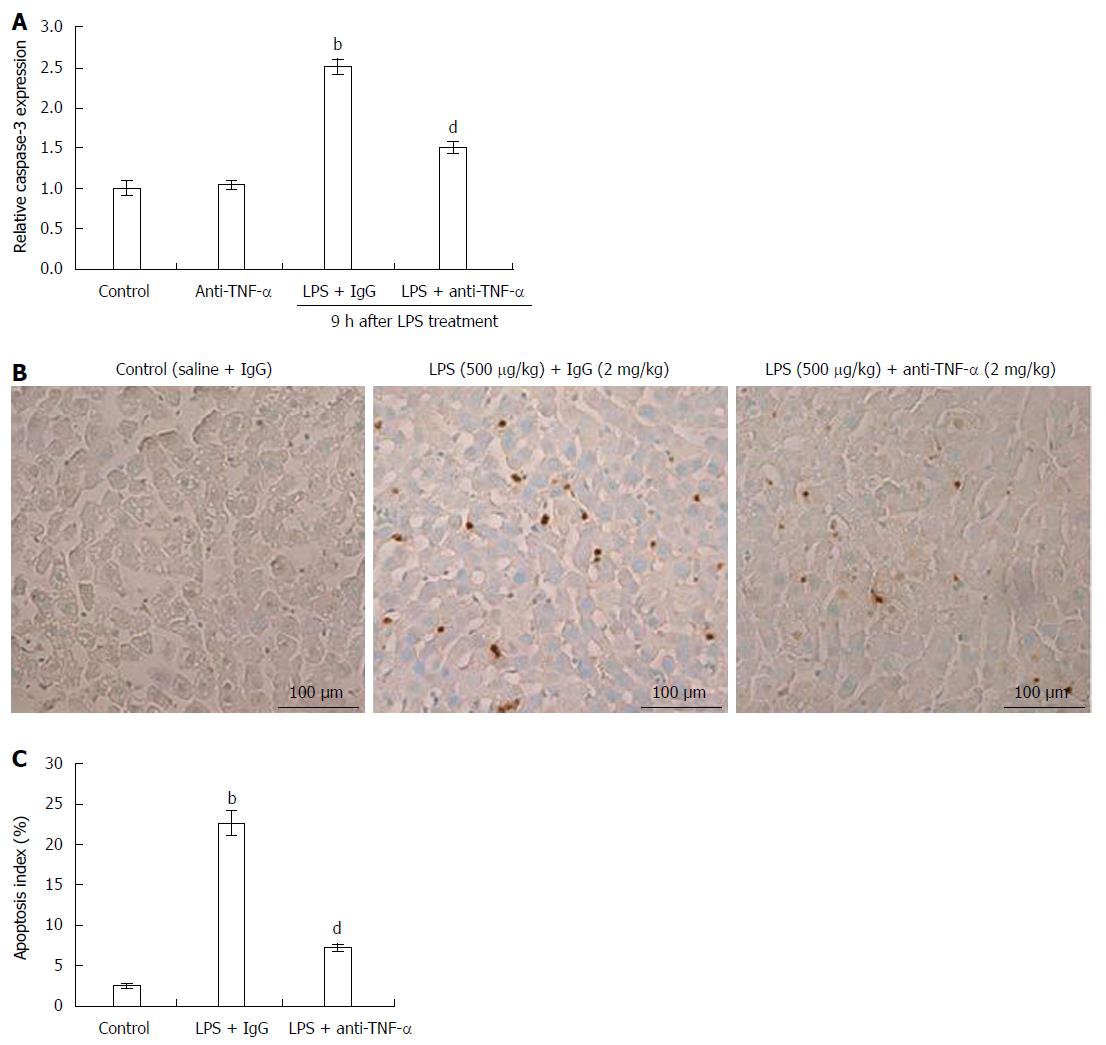
Liver apoptosis induced by LPS was confirmed by studying caspase-3 activation and TUNEL assay. Caspase-3 activation in LPS-treated rat livers increased 9 h after LPS + IgG injection, while the increased caspase-3 activity was significantly decreased by pretreatment with anti-TNF-α (Figure 2A). Typical TUNEL results are shown in Figure 2B and C. In the livers of LPS + IgG-treated rats, approximately 25% of hepatocytes nuclei were clearly stained 9 h after injection, whereas 2% positive changes were observed in control saline + IgG rat livers. Although IgG did not suppress an increase in the number of apoptotic hepatocytes, the addition of anti-TNF-α significantly inhibited the appearance of hepatocyte apoptosis (Figure 2B and C).
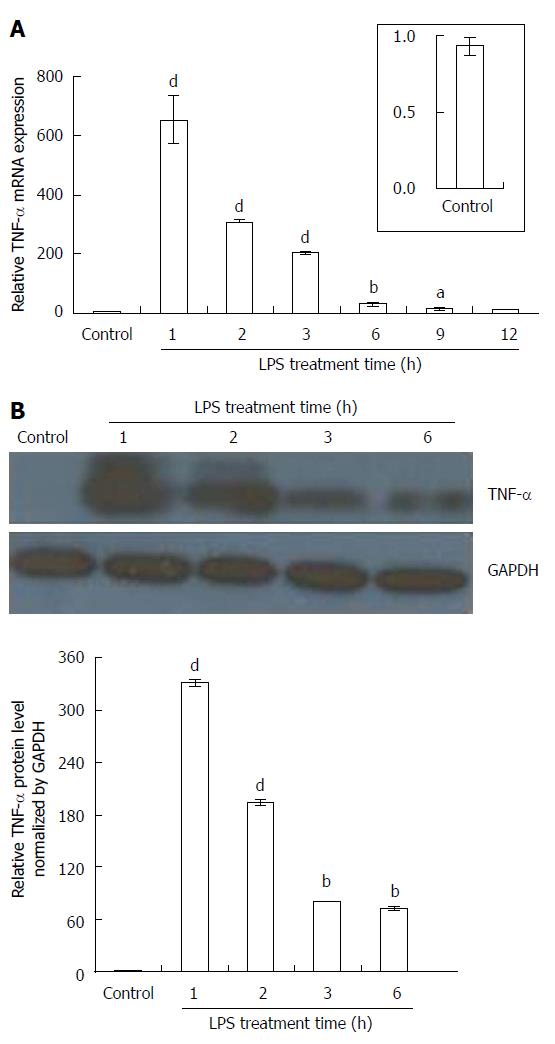
In unstimulated KCs (control saline + IgG), TNF-α was hardly detected; however, KCs treated with LPS (100 ng/mL) + IgG (500 ng/mL) showed marked production of TNF-α at 1 h after addition, and then a gradual decrease in mRNA and protein level (Figure 3A and B). Although the expression of TNF-α mRNA in LPS-treated KCs was similar to that obtained in LPS-treated rat livers, there was even stronger signal intensity for TNF-α protein in the former, appearing as a clearly detectable band 6 h after LPS administration (Figure 3B).
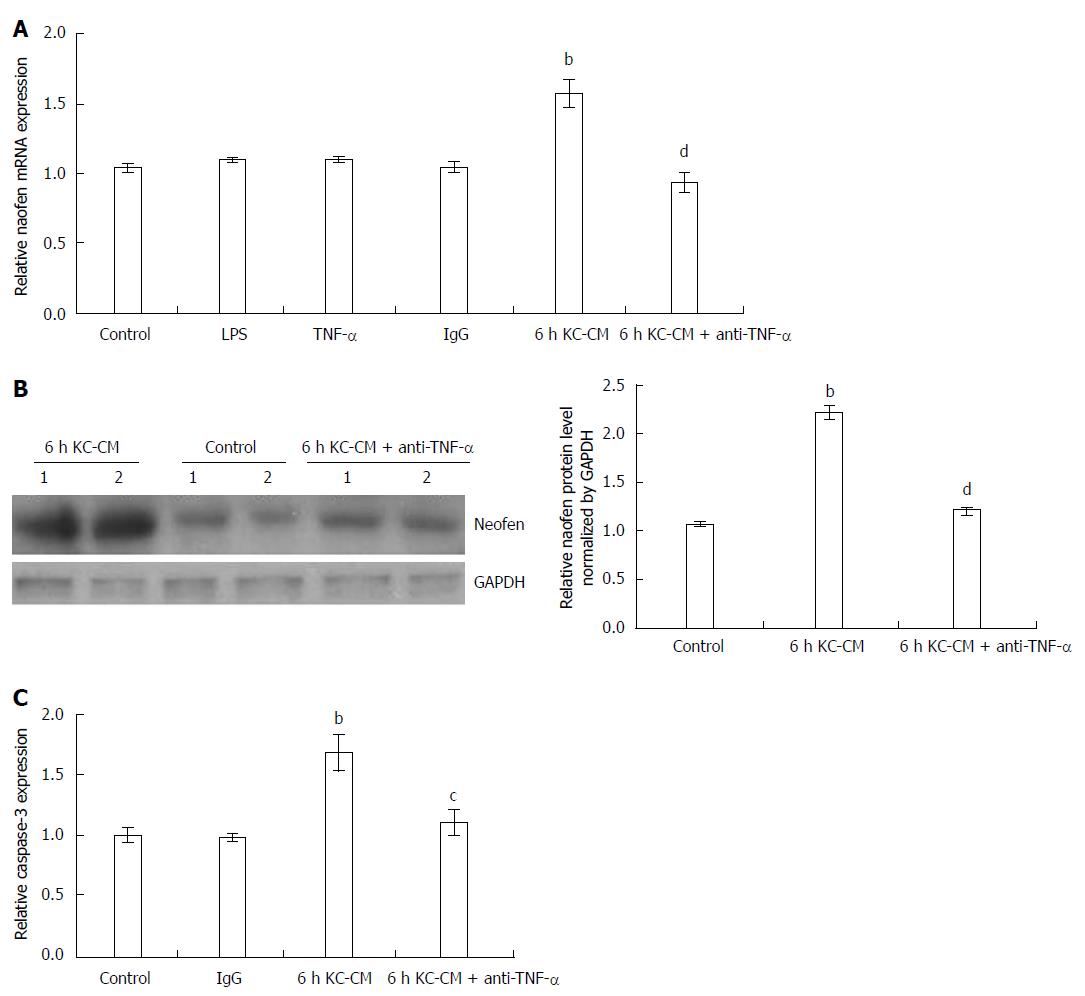
When LPS alone was added to hepatocytes (Figure 4A) or KCs, no change in naofen was observed (data not shown). As previously reported[19], KC-CM treated with LPS (100 ng/mL) for 3 h significantly increased naofen expression in hepatocytes, and extension of the LPS treatment time to 6 h had a stronger effect. In the following experiments, KC-CM treated with LPS for 6 h was used.
However, it was surprising that TNF-α alone did not enhance naofen expression in hepatocytes (Figure 4A). We have showed that anti-TNF-α antibody inhibits liver apoptosis induced by LPS (Figure 2), therefore, we studied the effect of anti-TNF-α antibody on KC-CM-induced naofen expression. As expected, pretreatment with 500 ng/mL anti-TNF-α antibody almost completely inhibited the increase of naofen induced by KC-CM (Figure 4A and B). An irrelevant antibody conferred no effect, suggesting the possible participation of TNF-α in the induction of naofen.
LPS alone did not affect caspase-3 activity, but in hepatocytes incubated with KC-CM for 6 h, caspase-3 activity significantly increased, which was clearly inhibited by pretreatment with 500 ng/mL anti-TNF-α (Figure 4C). These findings suggested that liver injury caused by LPS depended on TNF-α released from activated KC.
The results obtained in the present study demonstrated that LPS induced both naofen and TNF-α expression in rat liver. Naofen promotes TNF-α-mediated apoptosis of hepatocytes by activating caspase-3 in LPS-treated rats. In vitro, hepatocyte apoptosis caused by LPS was mediated by TNF-α, which was released from KCs in the presence of LPS, induced naofen expression and activated caspase-3. Our data suggested that hepatocyte apoptosis induced by KC-CM was associated with an increase in naofen expression (Figure 4), which was consistent with the results obtained in LPS-treated rats (Figure 1). Furthermore, naofen siRNA inhibited the increase in naofen protein induced by 6 h KC-CM, and naofen-siRNA also prevented KC-CM-induced caspase-3 activation in a previous study[19]. These results coincided with our recent data that naofen overexpression enhanced apoptosis by activating caspase-3 in HEK293 cells and, in contrast, naofen-siRNA inhibited TNF-α-induced caspase-3 activation and apoptosis[18]. Such results suggest that naofen is also involved in hepatocyte apoptosis induced by LPS-activated TNF-α. Previously, Morikawa et al[23] demonstrated that the injection of LPS and D-galactosamine into mice caused apoptosis in the kidneys, thymus, spleen, and lymph nodes besides the liver, whereas our findings verified that the increase in naofen induced by LPS was limited to the liver, and was not found in the kidneys, thymus or spleen (data not shown). This indicates that naofen, in LPS treated rats, may only make a limited contribution to liver injury.
Neither LPS nor TNF-α alone affected the expression of naofen in KCs or hepatocytes, whereas KC-CM significantly increased naofen expression in hepatocytes (Figure 4), indicating that the increase in naofen in the liver caused by LPS may be closely associated with KCs. As previously reported, the liver injury caused by LPS was dependent on KC activation, as demonstrated both in vitro and in vivo[8,9,24]. Intercellular signal transduction between KCs and hepatocytes has now been proposed, possibly mediated by cytokines such as TNF-α and IL, and inflammatory mediators such as eicosanoids, NO, and/or reactive oxygen species[6-9,25]. In particular, TNF-α has been shown to be an important mediator of LPS-induced apoptosis of hepatocytes[10,23,24]. The present study showed that LPS markedly enhanced TNF-α production in KCs in a time-dependent manner (Figure 4). It was noted that the time course of TNF-α expression in LPS-activated KCs accorded with that in LPS-treated rat livers (Figures 1 and 4), suggesting that LPS-induced TNF-α production in the liver may be ascribed to KCs, but not to hepatocytes. Furthermore, the increased naofen expression in LPS-treated rats, as well as the effects of KC-CM on naofen expression in hepatocytes, was clearly blocked by pretreatment with anti-TNF-α antibody (Figures 1 and 4), suggesting that TNF-α may play an important role in naofen expression. Regarding the little effect of TNF-α alone on naofen expression in hepatocytes, other unknown mediators may be associated with TNF-α, such as IL-1β, IL-6, IL-8, platelet-activating factor or NO[6-8,26]. Inhibitors of nuclear factor (NF)-κB may also be involved because blocking TNF-α-induced NF-κB activation in primary hepatocytes[27] or the liver in vivo[28] converts the hepatocellular TNF-α response from proliferation to apoptosis. In order to identify the nature of these unknown mediators, the effects on naofen expression of combination of TNF-α with IL-1, IL-6 and interferon-γ (10 ng/mL each) or inhibitors of NF-κB, such as BAY 11-7082 and DHMEQ, have been examined. However, combination with TNF-α or metabolites of TNF-α treated with trypsin failed to enhance naofen expression in primary hepatocytes (data not shown). It has been reported that the trend from TNF-α production to subsequent hepatocyte apoptosis may contribute to the development of several inflammatory liver diseases, including viral hepatitis, alcoholic liver disease, Wilson’s disease, drug-induced liver failure, and ischemia/reperfusion liver damage[7-10,24]. Identification of the relationship between TNF-α and naofen in liver injury may contribute to understanding the pathophysiological roles of apoptosis in liver diseases.
As previously reported, naofen is overexpressed in hepatocytes and markedly downregulates the expression of Bcl-2 and Bcl-xL, which is accompanied by the release of cytochrome c from mitochondria, resulting in caspase-3 activation[19]. Bcl-2 and Bcl-xL have critical roles in mitochondrial apoptotic signaling, through the controlled release of cytochrome c in hepatocytes[8,15]. This suggests that naofen is an upstream signal of Bcl-2 and Bcl-xL, consequently inducing the mitochondrial apoptotic pathway. Translocation of cytochrome c from mitochondria to cytosol has already been reported by many investigators, which forms a complex of Apaf-1 and procaspase-9, leading to the activation of caspase-9, followed by activation of downstream caspase-3 and development of hepatocyte apoptosis[13-15]. Likewise, previous studies have demonstrated that LPS-activated KCs also stimulate the apoptosis of hepatic stellate cells by activating caspases-9, -3 and -8[26,29]. Most importantly, naofen siRNA reverses KC-CM-induced responses, resulting in prevention of the decrease in Bcl-2 and Bcl-xL expression and increase in capase-3 activity[19]. Overall, naofen may act on the mitochondrial pathway in the KC-CM-induced apoptosis of hepatocytes. Therefore, it is possible that naofen is an intracellular mediator involved in TNF-α-mediated apoptosis of hepatocytes, and may be relevant to the investigation on LPS-induced hepatic injury.
In conclusion, naofen may be involved in part in LPS-induced hepatocyte apoptosis, which is mediated by mediators including TNF-α released from KCs. Naofen elicits inhibition of the expression of Bcl-2 and Bcl-xL, releasing cytochrome c from mitochondria, and activating caspase-3, finally leading to apoptosis of hepatocytes. Although the precise molecular mechanisms of LPS-mediated hepatocyte apoptosis are still incompletely defined, LPS-induced apoptotic mechanisms in relation to naofen may be relevant to understanding clinical endotoxin or septic shock, and offer a new approach to therapeutic applications.
Lipopolysaccharide (LPS) has no direct pathogenic effect in hepatocytes, but the apoptosis and inflammatory responses of the liver may be attributed mainly to the effusion of potent inflammatory mediators, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, and/or IL-10, from Kupffer cells (KCs). Among these mediators, TNF-α has been emphasized as a candidate for apoptosis and liver injury, as well as for infiltration of inflammatory cells. However, no clear mechanisms of LPS-induced hepatic damage have been demonstrated. Naofen, a WD40-repeat protein, may reduce NO synthesis or participate in TNF-α-induced apoptosis of HEK293 cells, but bupivacaine induces apoptosis independently of naofen expression. Whether naofen participates in TNF-α-mediated hepatocyte apoptosis in LPS-treated rats has not been investigated to date.
Naofen was recently found as an intracellular protein reactive to anti-verotoxin II antibody and classified in the aspartate-tryptophan (WD) 40-repeat protein family. The research hotspot is whether naofen participates in TNF-α-induced hepatocyte apoptosis in LPS-treated rats.
This study verified that the increase in naofen induced by LPS was limited to the liver, and was not found in the kidneys, thymus or spleen, indicating that naofen, in LPS treated rats, may only have a limited contribution to liver injury. So, the correlating mechanisms were evaluated, utilizing primary cultures of KCs and hepatocytes. Moreover, the action of LPS on apoptosis of hepatocytes was not induced by direct effects, but rather via an indirect pathway through the enhanced release of TNF-α from KCs. LPS or TNF-α alone did not elicit apoptosis of primary hepatocytes, or affect naofen expression or caspase-3 activation in primary hepatocytes. To overcome these disadvantages, combination of TNF-α with IL-1, IL-6 and interferon-γ or inhibitors of nuclear factor-κB was investigated. Conditioned medium from LPS-treated KCs (KC-CM) and anti-TNF-α antibody were used for further experiments. In this study, elevation of naofen expression and caspase-3 activity in LPS-treated rats was abrogated by pretreatment with anti-TNF-α antibody. Furthermore, the KC-CM-induced increase in naofen expression and caspase-3 activity was blocked by anti-TNF-α antibody. Naofen may be involved in part in LPS-induced hepatocyte apoptosis, which is mediated by mediators including TNF-α released from KCs.
The roles of naofen in LPS-induced apoptosis may be relevant to the understanding of clinical endotoxin/septic shock, and offer a new approach to therapeutic applications.
LPS is a major structural component of the outer membrane of Gram-negative bacteria, and causes hepatic dysfunction, possibly associated with apoptosis of hepatocytes, which is mediated by inflammatory substances, such as TNF-α released from KCs. Naofen was recently identified as an intracellular protein reactive to anti-verotoxin II antibody and classified in the WD 40-repeat protein family.
This was a follow-up study of previous studies published by the authors. The study was novel and well designed. The study investigated the role of naofen in TNF-α-mediated apoptosis of hepatocytes induced by LPS. It was concluded that TNF-α released from KCs treated with LPS may induce hepatic naofen expression and then stimulate hepatocellular apoptosis through activation of caspase-3.
P- Reviewers: Ricci-Vitiani L, Tarantino G, Vinken M, Zhang HL S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
| 1. | Morrison DC, Danner RL, Dinarello CA, Munford RS, Natanson C, Pollack M, Spitzer JJ, Ulevitch RJ, Vogel SN, Mc Sweegan E. Bacterial endotoxins and pathogenesis of Gram-negative infections: current status and future direction. Innate Immun. 1994;1:71-83. [RCA] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Nolan JP. Endotoxin, reticuloendothelial function, and liver injury. Hepatology. 1981;1:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 246] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Ulevitch RJ, Mathison JC, Schumann RR, Tobias PS. A new model of macrophage stimulation by bacterial lipopolysaccharide. J Trauma. 1990;30:S189-S192. [PubMed] |
| 4. | Jirillo E, Caccavo D, Magrone T, Piccigallo E, Amati L, Lembo A, Kalis C, Gumenscheimer M. The role of the liver in the response to LPS: experimental and clinical findings. J Endotoxin Res. 2002;8:319-327. [PubMed] |
| 5. | Nolan JP, Camara DS. Intestinal endotoxins as co-factors in liver injury. Immunol Invest. 1989;18:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Higuchi H, Gores GJ. Mechanisms of liver injury: an overview. Curr Mol Med. 2003;3:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641-1654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 412] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 8. | Tilg H. Cytokines and liver diseases. Can J Gastroenterol. 2001;15:661-668. [PubMed] |
| 9. | Hoebe KH, Witkamp RF, Fink-Gremmels J, Van Miert AS, Monshouwer M. Direct cell-to-cell contact between Kupffer cells and hepatocytes augments endotoxin-induced hepatic injury. Am J Physiol Gastrointest Liver Physiol. 2001;280:G720-G728. [PubMed] |
| 10. | Hamada E, Nishida T, Uchiyama Y, Nakamura J, Isahara K, Kazuo H, Huang TP, Momoi T, Ito T, Matsuda H. Activation of Kupffer cells and caspase-3 involved in rat hepatocyte apoptosis induced by endotoxin. J Hepatol. 1999;30:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Teoh N, Field J, Sutton J, Farrell G. Dual role of tumor necrosis factor-alpha in hepatic ischemia-reperfusion injury: studies in tumor necrosis factor-alpha gene knockout mice. Hepatology. 2004;39:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Zhou W, Zhang Y, Hosch MS, Lang A, Zwacka RM, Engelhardt JF. Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia/reperfusion. Hepatology. 2001;33:902-914. [PubMed] |
| 13. | Neuman MG. Apoptosis in liver disease. Rom J Gastroenterol. 2002;11:3-7. [PubMed] |
| 14. | Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43:S31-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 499] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 15. | Guicciardi ME, Gores GJ. Apoptosis as a mechanism for liver disease progression. Semin Liver Dis. 2010;30:402-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Feng GG, Yamada M, Wongsawatkul O, Li C, Huang L, An J, Komatsu T, Fujiwara Y, Naohisa I. Role of naofen, a novel WD repeat-containing protein, in reducing nitric oxide-induced relaxation. Clin Exp Pharmacol Physiol. 2008;35:1447-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Sato Y, Feng GG, Huang L, Fan JH, Li C, An J, Tsunekawa K, Kurokawa S, Fujiwara Y, Komatsu T. Enhanced expression of naofen in kidney of streptozotocin-induced diabetic rats: possible correlation to apoptosis of tubular epithelial cells. Clin Exp Nephrol. 2010;14:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Feng GG, Li C, Huang L, Tsunekawa K, Sato Y, Fujiwara Y, Komatsu T, Honda T, Fan JH, Goto H. Naofen, a novel WD40-repeat protein, mediates spontaneous and tumor necrosis factor-induced apoptosis. Biochem Biophys Res Commun. 2010;394:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Fan JH, Feng GG, Huang L, Tsunekawa K, Honda T, Katano Y, Hirooka Y, Goto H, Kandatsu N, Ando K. Role of naofen in apoptosis of hepatocytes induced by lipopolysaccharide through mitochondrial signaling in rats. Hepatol Res. 2012;42:696-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Kohira T, Matsumoto K, Ichihara A, Nakamura T. Identification of a biologically functional novel IL-1 beta-specific receptor on adult rat hepatocytes. J Biochem. 1993;114:658-662. [PubMed] |
| 21. | Olynyk JK, Clarke SL. Isolation and primary culture of rat Kupffer cells. J Gastroenterol Hepatol. 1998;13:842-845. [PubMed] |
| 22. | Xu J, Yeh CH, Chen S, He L, Sensi SL, Canzoniero LM, Choi DW, Hsu CY. Involvement of de novo ceramide biosynthesis in tumor necrosis factor-alpha/cycloheximide-induced cerebral endothelial cell death. J Biol Chem. 1998;273:16521-16526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Morikawa A, Sugiyama T, Kato Y, Koide N, Jiang GZ, Takahashi K, Tamada Y, Yokochi T. Apoptotic cell death in the response of D-galactosamine-sensitized mice to lipopolysaccharide as an experimental endotoxic shock model. Infect Immun. 1996;64:734-738. [PubMed] |
| 24. | Bradham CA, Plümpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol. 1998;275:G387-G392. [PubMed] |
| 25. | Liu D, Li C, Chen Y, Burnett C, Liu XY, Downs S, Collins RD, Hawiger J. Nuclear import of proinflammatory transcription factors is required for massive liver apoptosis induced by bacterial lipopolysaccharide. J Biol Chem. 2004;279:48434-48442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Oh SH, Lee BH. A ginseng saponin metabolite-induced apoptosis in HepG2 cells involves a mitochondria-mediated pathway and its downstream caspase-8 activation and Bid cleavage. Toxicol Appl Pharmacol. 2004;194:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Xu Y, Bialik S, Jones BE, Iimuro Y, Kitsis RN, Srinivasan A, Brenner DA, Czaja MJ. NF-kappaB inactivation converts a hepatocyte cell line TNF-alpha response from proliferation to apoptosis. Am J Physiol. 1998;275:C1058-C1066. [PubMed] |
| 28. | Iimuro Y, Nishiura T, Hellerbrand C, Behrns KE, Schoonhoven R, Grisham JW, Brenner DA. NFkappaB prevents apoptosis and liver dysfunction during liver regeneration. J Clin Invest. 1998;101:802-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 357] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 29. | Fischer R, Cariers A, Reinehr R, Häussinger D. Caspase 9-dependent killing of hepatic stellate cells by activated Kupffer cells. Gastroenterology. 2002;123:845-861. [PubMed] |