Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. Apr 7, 2014; 20(13): 3572-3581
Published online Apr 7, 2014. doi: 10.3748/wjg.v20.i13.3572
Published online Apr 7, 2014. doi: 10.3748/wjg.v20.i13.3572
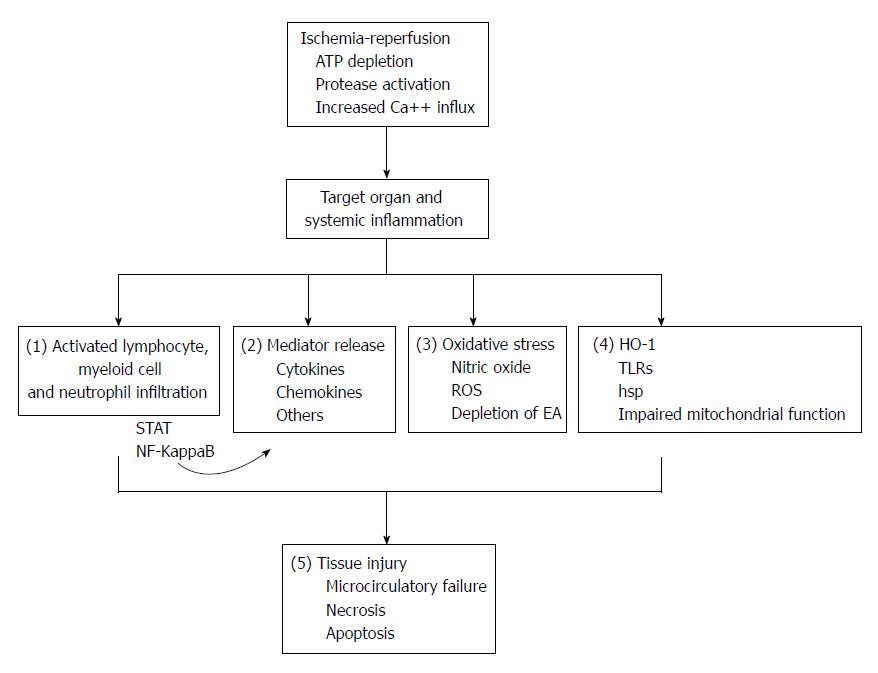
Figure 1 Pathophysiology of ischemia-reperfusioin injury and mechanisms modulated by remote ischemic preconditioning.
There are multiple pathways involved in the pathophysiology of ischemia-reperfusion (IR) injury. This leads to end organ damage as discussed in the text. Inflammatory cell infiltration and activation during IR is driven by multiple signaling pathways and lead to tissue inflammation. (1) Remote ischemic preconditioning (RIP) can modulate inflammatory cell infiltration and activity. Inflammatory cells and resident cells produce inflammatory cytokines and chemokines, partly in response to signal transducers and activators of transcription factor (STAT) and nuclear factor kappa B (NF-Kappa B) signaling; (2) RIP can reduce cytokine and chemokine production and modulate STAT activity. Local and infiltrating inflammatory cells produce reactive oxygen species (ROS) that deplete endogenous antioxidants (EA), resulting in tissue distruction; (3) RIP reduces production of ROS and increases levels of EA. During IR, heme-oxygenase-1 (HO-1) and heat shock proteins (hsp) are upregulated, Toll-like receptors (TLRs) are activated and mitochondrial function is disrupted; (4) RIP modulates TLR signaling, protects mitochondria and regulates HO-1 production. All of these events lead to microcirculatory failure and finally cellular apoptosis; and (5) RIP preserves microcirculation and reduces pro-apoptotic signals. ATP: Adenosine triphosphate.
- Citation: Camara-Lemarroy CR. Remote ischemic preconditioning as treatment for non-ischemic gastrointestinal disorders: Beyond ischemia-reperfusion injury. World J Gastroenterol 2014; 20(13): 3572-3581
- URL: https://www.wjgnet.com/1007-9327/full/v20/i13/3572.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i13.3572