Published online Apr 7, 2014. doi: 10.3748/wjg.v20.i13.3615
Revised: December 19, 2013
Accepted: January 3, 2014
Published online: April 7, 2014
AIM: To investigate the efficacy of a standard triple therapy (comprising rabeprazole, clarithromycin, and amoxicillin) for Helicobacter pylori (H. pylori) eradication, noting factors that influence the outcome and documenting any adverse events.
METHODS: Following institutional ethical approval, fifty consecutive and consenting symptomatic patients with evidence of H. pylori infection by either a positive urea breath test (UBT) and/or a campylobacter-like organism test who presented to the Gastroenterology clinic of Lagos State University Teaching Hospital between 2012 and 2013 were recruited into the study. Patients were openly randomized to either a 7-d or a 10-d regimen of amoxicillin 1 g, clarithromycin 500 mg and rabeprazole 20 mg twice daily. The extent of symptom resolution was noted following the treatment, and at the end of one month after the completion of treatment, a repeat UBT was performed in each patient to document the eradication of the infection. All data (demographics, symptoms, and eradication rates) were collated and analyzed with SPSS version 18.
RESULTS: Forty-seven patients completed the study (three were excluded from the analysis for breaching the study protocol). The patients included 18 males and 29 females within the age range of 13-80 years (mean 43.7, SD 16.8). The clinical features of the study subjects were dyspepsia, reflux symptoms and features of gastrointestinal bleeding. The average eradication rate was 87.2%. Eighteen subjects were enrolled in the 7-d arm, while 29 were in the 10-d arm. There was no statistically significant difference in the age or sex distributions of the two arms. There was no significant advantage of the 10-d treatment duration over the 7-d duration (P = 0.78), and the eradication outcomes were not influenced by the gender or age of the subjects. No adverse effects were reported in either arm.
CONCLUSION: The triple therapy regime, employing a combination of amoxicillin, clarithromycin and rabeprazole, showed great efficacy and safety in the eradication of H. pylori, and this outcome was not influenced by gender or age. No difference was observed between the 7-d and 10-d regimens.
Core tip:Helicobacter pylori (H. pylori) infection is widespread in Nigeria, along with the associated risk of serious gastroduodenal diseases, including gastric cancer. The use of different eradication therapies for H. pylori is commonplace, as no consensus exists regarding the optimal treatment regimen, despite guidelines and recommendations. This lack of consensus is most likely due to the lack of evidence-based data, and the findings in this report may help to fill this gap.
-
Citation: Onyekwere CA, Odiagah JN, Igetei R, Duro Emanuel AO, Ekere F, Smith S. Rabeprazole, clarithromycin, and amoxicillin
Helicobacter pylori eradication therapy: Report of an efficacy study. World J Gastroenterol 2014; 20(13): 3615-3619 - URL: https://www.wjgnet.com/1007-9327/full/v20/i13/3615.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i13.3615
Helicobacter pylori (H. Pylori) are gram-negative spiral bacteria that reside within the gastric mucosa and are implicated in the etiopathogenesis of a number of gastroduodenal diseases[1]. Although many infected persons do not experience clinically meaningful sequelae, many others develop serious gastrointestinal-related conditions, including gastric cancer. There is a high prevalence of this infection in developing countries, including Nigeria, where reports indicate a prevalence ranging from 45% to 64% among adult dyspeptics, depending on the diagnostic criteria employed[2,3]. Several guidelines for the eradication of this infection have been published by different professional groups in different geographical settings, including the European consensus report and the World Gastroenterology Organization[4,5]. The aim of H. pylori eradication is to reduce the lifetime risk of peptic ulcer disease and possibly of gastric cancer. The test and treat strategy has been advocated in a setting of high H. Pylori prevalence (> 20%) and low gastric cancer risk[6]. The urea breath test (UBT), which uses essentially (13C) urea, remains the best test to diagnose an H. pylori infection due to its high accuracy and ease of use[7]. When endoscopy is performed, biopsy-based tests, such as the rapid urease test, histology and culture, can be carried out. Hitherto, the UBT as a diagnostic tool has not been widely employed in Nigerian settings; poor accessibility, which includes costs and availability, is partially responsible for this scenario. The current H. Pylori treatment guidelines suggest the use of triple therapy [usually a proton-pump inhibitor (PPI), amoxicillin, and clarithromycin (standard triple therapy)] as the first-line therapy for a duration ranging from 7 to 14 d[6]. Other combinations involving the use of metronidazole may be used, depending on the local resistance pattern. Extending the duration of treatment from 7 to 10-14 d has been shown to increase the success rate of eradication[8]. Other first-line therapies include the quadruple therapy, which involves the addition of a bismuth compound; although this approach is cheaper while having a similar efficacy, more side effects are associated with poor compliance[5] when compared with the PPI. These side effects were later associated with a low rate of transient and asymptomatic serum aminotransferase elevations and represent a rare cause of clinically apparent liver injury[9]. The choice of an eradication regimen is often influenced by such factors as efficacy, adverse effects, cost, compliance, and local resistance patterns. Earlier studies indicated a high rate of metronidazole and amoxicillin resistance in our setting[10]. The empirical use of eradication therapy has become commonplace in our setting, following the National Institutes of Health consensus statement[11] on testing and treatment strategies for H. pylori. Often, no retesting is performed to ensure that the eradication of the infection has been accomplished following treatment. Presently, no consensus exists on the choice of an optimal eradication regime in Nigeria, despite the guidelines[5] and recommendations made in view of differing antimicrobial sensitivity patterns across and within different geographical settings. We set out to investigate the efficacy of the standard triple therapy regimen (comprising rabeprazole, clarithromycin and amoxicillin).
From June 2012 to August 2013, we recruited subjects into this randomized, controlled trial, carried out at the Gastroenterology Unit of The Lagos State University Teaching Hospital, Ikeja Nigeria.
Inclusion criteria were the following: (1) persons who presented to the unit with upper gastrointestinal (GI) symptoms, such as dyspepsia, upper GI bleeding, or gastroesophageal reflux symptoms (heartburn); and (2) persons with evidence of H. pylori infection. H. pylori infection was said to be present based on either a positive UBT or campylobacter-like organism test. The UBT test was carried out using the Kibion heliprobe machine and involved the ingestion of 13C labeled urea (helicap) after six hours of fasting and the subsequent exhalation into a breath card. The amount of exhaled 13C is measured by the heliprobe breath analyzer. The details of measurement are given according to the manufacturer’s protocol[12].
The exclusion criteria are stated below: (1) persons with upper GI symptoms who were already on antisecretory drugs, including PPI or antibiotics; (2) persons with upper GI symptoms who were acutely ill and required hospitalization; and (3) persons who refused consent. Using an estimated eradication rate of 85% based on previous reports[13] of triple therapy and a degree of accuracy of 0.1, a sample size of 49 was calculated using the formula N = Z2 pq/d2, where N = the required sample size, Z = the standard normal deviation (usually set at 1.96 to correspond to the 95%CI), p = the proportion in the target population estimated to have a particular characteristic, q = 1 - p, d = the degree of accuracy desired set at 0.1. Following institutional ethical approval and written informed consent, the study’s participants were consecutively recruited. Alternate patients were openly randomized to either a 7- or 10-d course of the eradication regimen. They were instructed not to use any antibiotic or other antisecretory drugs, including proton pump inhibitors, during the study period and to report any adverse reactions to the principal investigator, whose contact information was shared with all of the participating subjects. The 10-d regimen arm involved use of rabeprazole 20 mg, amoxicillin 1 g and Clarithromycin 500 mg twice daily for 10 d, while the 7-d treatment arm used the same combination of drugs and dosages but for 7 d. At the end of 1 mo following the completion of the treatment, a repeat UBT was performed on each patient to document the eradication of the infection.
The outcome parameters for this study are symptomatic improvement and successful eradication. Symptomatic improvement refers to the resolution of the initial presenting symptoms. Successful eradication was said to be present if the repeat UBT at one month following the completion of treatment was negative.
This study were performed with SPP version 18. The test statistics used included Student’s t test for the comparison of quantitative data and the χ2 test for the comparison of qualitative variables. A P value of ≤ 0.05 was deemed significant.
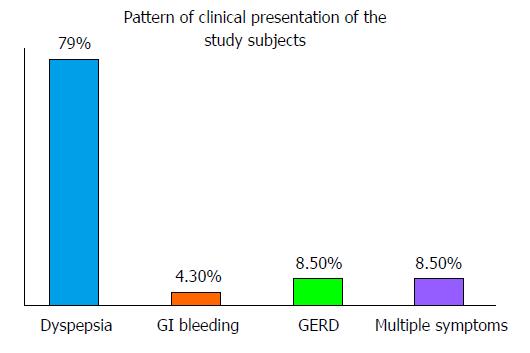
Forty-seven patients completed the study, as three of the patients (2 from the 7-d arm and 1 from the 10-d arm) dropped out of the study (2 for using other proton pump inhibitors and 1 for not adhering to the study protocol). Thus, the non-compliant patients were excluded from the analysis. The study group was made up of 18 males and 29 females within the age range 13-80 years (mean 43.7 SD 16.8). The clinical features of the study subjects are given in Figure 1. The average eradication rate was 87.2%.
Adverse effects. No adverse effects were reported in either arm. Table 1 illustrates the relationship between the outcome of the H. pylori eradication and the patients’ demographics (age and sex) and clinical presentations. There was no significant relationship between the age, sex or pattern of clinical presentation among those subjects with successful H. Pylori eradication.
| Successful eradication | Yes | No | Total number of subjects | |
| Age of the patient vs eradication outcome | ||||
| Age grouping of patient | 0-39 yr | 19 | 3 | 22 |
| 40-59 yr | 14 | 1 | 15 | |
| ≥ 60 yr | 8 | 2 | 10 | |
| Total | 41 (mean age 41 ± 17) | 6 (mean age 48 ± 16) | ||
| P value 0.611 (χ2 = 0.986) | ||||
| Gender vs outcome of eradication therapy | ||||
| Gender | Male | 16 | 2 | 18 |
| Female | 25 | 4 | 29 | |
| Total | 41 | 6 | 47 | |
| P value 0.582 (χ2 = 0.072) | ||||
| Patients’ clinical presentation vs outcomes of eradication treatment | ||||
| Clinical presentation | Dyspepsia | 34 | 3 | 37 |
| GERD | 2 | 2 | 4 | |
| GI bleeding | 2 | 0 | 2 | |
| Multiple presentation | 3 | 1 | 4 | |
| Total | 41 | 6 | 47 | |
| P value 0.088 9 (χ2 = 6.531) | ||||
Eighteen subjects were in the seven-day arm, while 29 were in the 10-d arm. The age and sex distributions of the subjects in the two arms are given in Table 2. There was no statistically significant difference in the age and sex distribution or in the rate of successful H. Pylori eradication between the two arms.
| Factor | Seven-day arm | Ten-day arm | P value |
| Age range (mean ± SD) | 13-71 (41.56 ± 16.94) | 18-80 (44.93 ± 16.94) | 0.504 |
| Sex | 0.581 | ||
| Male | 6 (33.3) | 12 (41.4) | |
| Female | 12 (66.7) | 17 (58.6) | |
| Symptomatic improvement | 0.215 | ||
| Yes | 17 (94.4) | 29 (100.0) | |
| No | 1 (5.6) | 0 (0.0) | |
| Successful eradication | 0.789 | ||
| Yes | 16 (88.9) | 25 (86.2) | |
| No | 2 (11.1) | 4 (13.8) | |
Infection with H. pylori continues to be a cause for concern, and the search for an optimal therapy continues due to the changing antibiotic sensitivity patterns in different geographic settings. Triple therapy with an antisecretory drug and two antibiotics (amoxicillin, metronidazole or clarithromycin) has often been advocated as the first-line therapy; the choice of the antibiotics varies, depending on local sensitivity patterns[6]. Our study has shown an overall symptom improvement of over 95% and a H. pylori eradication rate of over 87%, which is a little less than the 90% per protocol recommended by World Gastroenterology Organisation guidelines[5]. This rate is also slightly lower than the rates obtained by Sokwala et al[13] in a Kenyan population employing same antibiotics but another PPI (Esomeprazole). However, our rate is better than the 85% reported by Wong et al[14] in their multi-center study of a triple therapy, employing the same antibiotics but with omeprazole in Asians and Africans. Still, it has been reported that, in clinical trials, failure to eradicate H. pylori is approximately 20% after first-line eradication therapy[15]. Antibiotic resistance patterns[10], due most likely to the abuse of drugs as antibiotics, are sold without prescriptions in our setting; this may partly explain our lower efficacy. The current report indicates a resistance of < 5% to tetracycline, < 30% to amoxicillin, < 16% to clarithromycin and 100% to metronidazole[16] in Lagos, Nigeria. This observed eradication rate, in any case, gives us some confidence that eradication was achieved in the majority of our treated patients through the use of this triple therapy combination. Still, in routine practice, post-treatment evaluation is hardly ever performed, contrary to the guidelines and recommendations[6]. The few (13%) patients who failed to respond to this regime were treated successfully with either a sequential therapy[17] or quadruple therapy[5]. Further, the eradication rates did not vary with the age, despite that previous reports[18] have suggested worse outcomes at younger ages. Equally, some reports have indicated that the failure of eradication is more common in the female sex[19]; however our findings did not suggest any gender predisposition. The pattern of clinical manifestation of the subjects had no relation to their outcome, although some reports suggested an association with the subjects’ gastric histology features[20].
We did not find any statistically significant differences in the eradication rates between those treated for 7 d (88.9%) and those treated for 10 d (86.2%), contrary to earlier reports[7]. Similar observations were made in two other reports involving African populations[12,14]. Although no consensus exists for the optimal duration of triple therapy in Africa, it is noteworthy that a number of European guidelines[4] favor 7-d therapies, as opposed to the extended 10 to 14 d treatment in America[21].
We were, however, constrained by our modest subject sample size due to the limited resources available to our investigators. Additionally, we did not investigate the effects of the subjects’ habits, such as smoking or alcohol consumption, on the eradication rate, although some reports[13] have indicated that these habits could affect the outcomes of the eradication treatment. The cost benefit analysis of this triple therapy regime was also not investigated, as this was not included into the study design.
The triple therapy regime, employing a combination of amoxicillin, clarithromycin and rabeprazole, has shown great efficacy and safety in the eradication of H. pylori, demonstrating an average eradication rate of 87.2%. The 10-d treatment duration showed no significant advantage over the 7-d duration, and the outcome was not influenced by the gender or age of the subjects.
We appreciate the support of Biofem pharmaceuticals and Solidum pharmaceuticals in carrying out this project, as well as the support of Dr. O. Ogbera for helping to proofread the manuscript.
Helicobacter pylori (H. pylori) infection is common in Sub-Saharan Africa, including Nigeria, along with its attendant consequences, including gastric cancer. Several guidelines have been recommended regarding an eradication therapy using a number of regimes. No consensus on such therapies exists in the face of changing anti-microbial sensitivity patterns.
Although post-treatment tests are recommended to ensure that H. pylori eradication is achieved, this is not often performed by practitioners. There is a need for an evidence-based, safe, and effective treatment regime in a setting with the widespread use of empirical H. pylori eradication.
This randomized trial has demonstrated the efficacy of a rabeprazole, amoxicillin and cIarithromycin triple regime in achieving a high eradication rate; additionally, a 7-d regime was shown to be just as effective as a 10-d regime. The outcomes of the treatment were not influenced by age or gender.
The study results suggest that the triple therapy, employing rabeprazole, amoxicillin and clarithromycin for seven days, can be used to treat H. pylori infections in Lagos, Nigeria.
The article is aim to investigate the efficacy of a triple therapy (comprising of Rabeprazole, Clarithromycin and Amoxicillin) and note factors influencing the outcome as well as document any adverse event. The manuscript is very well-written.
P- Reviewers: Amornyotin S, Ibrahim M, Pereira RD, Takeno S S- Editor: Cui XM L- Editor: A E- Editor: Ma S
| 1. | Marshall BJ. Helicobacter Foundation. Available from: http://www.helico.com viewed 2/12/13. [Cited in This Article: ] |
| 2. | Smith SI, Oyedeji KS, Arigbagbu A, Anomneze EE, Chibututu CC, Atimomo EA, Atoyebi A, Adesanya , A , Coker AO. Prevalence of H. pylori in patients with gastritis and peptic ulcer in Western, Nigeria. Biomed Lett. 1999;60:115-120. [Cited in This Article: ] |
| 3. | Jemilohun AC, Otegbayo JA, Ola SO, Oluwasola OA, Akere A. Prevalence of Helicobacter pylori among Nigerian patients with dyspepsia in Ibadan. Pan Afr Med J. 2010;6:18. [PubMed] [Cited in This Article: ] |
| 4. | Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1348] [Cited by in F6Publishing: 1295] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 5. | World gastroenterology organization. WGO Practice Guideline: Helicobacter pylori in Developing Countries. Available from: http://www.WGO viewed 09/09/13. [Cited in This Article: ] |
| 6. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1541] [Cited by in F6Publishing: 1497] [Article Influence: 124.8] [Reference Citation Analysis (4)] |
| 7. | Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection -- a critical review. Aliment Pharmacol Ther. 2004;20:1001-1017. [PubMed] [Cited in This Article: ] |
| 8. | Fallone CA, Barkun AN, Szilagyi A, Herba KM, Sewitch M, Martel M, Fallone SS. Prolonged treatment duration is required for successful Helicobacter pylori eradication with proton pump inhibitor triple therapy in Canada. Can J Gastroenterol. 2013;27:397-402. [PubMed] [Cited in This Article: ] |
| 9. | Smith SI, Oyedeji KS, Arigbabu AO, Atimomo C, Coker AO. High amoxycillin resistance in Helicobacter pylori isolated from gastritis and peptic ulcer patients in western Nigeria. J Gastroenterol. 2001;36:67-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Sandig C, Flechtenmacher C, Stremmel W, Eisenbach C. Pantoprazole induces severe acute hepatitis. Z Gastroenterol. 2011;49:207-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | US dept of Health and Human services. Helicobacter pylori in peptic ulcer disease. NIH consensus statement. 1994;12:1-23. [Cited in This Article: ] |
| 12. | Oztürk E, Yeşilova Z, Ilgan S, Arslan N, Erdil A, Celasun B, Ozgüven M, Dağalp K, Ovali O, Bayhan H. A new, practical, low-dose 14C-urea breath test for the diagnosis of Helicobacter pylori infection: clinical validation and comparison with the standard method. Eur J Nucl Med Mol Imaging. 2003;30:1457-1462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Sokwala A, Shah MV, Devani S, Yonga G. Helicobacter pylori eradication: A randomised comparative trial of 7-day versus 14-day triple therapy. S Afr Med J. 2012;102:368-371. [PubMed] [Cited in This Article: ] |
| 14. | Wong BC, Chang FY, Abid S, Abbas Z, Lin BR, Van Rensburg C, Chen PC, Schneider H, Simjee AE, Hamid SS. Triple therapy with clarithromycin, omeprazole, and amoxicillin for eradication of Helicobacter pylori in duodenal ulcer patients in Asia and Africa. Aliment Pharmacol Ther. 2000;14:1529-1535. [PubMed] [Cited in This Article: ] |
| 15. | Gisbert JP, Calvet X. Review article: the effectiveness of standard triple therapy for Helicobacter pylori has not changed over the last decade, but it is not good enough. Aliment Pharmacol Ther. 2011;34:1255-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Smith Stella. Nigerian Institute of Medical Research. Antibiotic Sensitivity patterns of helicobacter isolates from South West Nigeria: Personal communication; . [Cited in This Article: ] |
| 17. | Chung JW, Jung YK, Kim YJ, Kwon KA, Kim JH, Lee JJ, Lee SM, Hahm KB, Lee SM, Jeong JY. Ten-day sequential versus triple therapy for Helicobacter pylori eradication: a prospective, open-label, randomized trial. J Gastroenterol Hepatol. 2012;27:1675-1680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Treiber G, Wittig J, Ammon S, Walker S, van Doorn LJ, Klotz U. Clinical outcome and influencing factors of a new short-term quadruple therapy for Helicobacter pylori eradication: a randomized controlled trial (MACLOR study). Arch Intern Med. 2002;162:153-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Cai W, Zhou L, Ren W, Deng L, Yu M. Variables influencing outcome of Helicobacter pylori eradication therapy in South China. Helicobacter. 2009;14:91-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Georgopoulos SD, Ladas SD, Karatapanis S, Mentis A, Spiliadi C, Artikis V, Raptis SA. Factors that may affect treatment outcome of triple Helicobacter pylori eradication therapy with omeprazole, amoxicillin, and clarithromycin. Dig Dis Sci. 2000;45:63-67. [PubMed] [Cited in This Article: ] |
| 21. | Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825. [PubMed] [Cited in This Article: ] |