Published online Mar 14, 2014. doi: 10.3748/wjg.v20.i10.2515
Revised: October 24, 2013
Accepted: November 12, 2013
Published online: March 14, 2014
Processing time: 166 Days and 13.8 Hours
Chronic liver inflammation drives hepatic fibrosis, and current immunosuppressive, anti-inflammatory, and anti-viral therapies can weaken this driver. Hepatic fibrosis is reversed, stabilized, or prevented in 57%-79% of patients by conventional treatment regimens, mainly by their anti-inflammatory actions. Responses, however, are commonly incomplete and inconsistently achieved. The fibrotic mechanisms associated with liver inflammation have been clarified, and anti-fibrotic agents promise to improve outcomes as adjunctive therapies. Hepatitis C virus and immune-mediated responses can activate hepatic stellate cells by increasing oxidative stress within hepatocytes. Angiotensin can be synthesized by activated hepatic stellate cells and promote the production of reactive oxygen species. Anti-oxidants (N-acetylcysteine, S-adenosyl-L-methionine, and vitamin E) and angiotensin inhibitors (losartin) have had anti-fibrotic actions in preliminary human studies, and they may emerge as supplemental therapies. Anti-fibrotic agents presage a new era of supplemental treatment for chronic liver disease.
Core tip: The prevention of hepatic fibrosis and the reversal of cirrhosis are now achievable objectives in the management of chronic liver disease. Conventional immunosuppressive, anti-inflammatory, and anti-viral therapies can accomplish these outcomes by reducing liver damage, suppressing hepatic inflammation, and eliminating etiological agents, but they do so inconsistently and indirectly. The continuing clarification of pro-fibrotic mechanisms affords opportunities to design site-specific, anti-fibrotic interventions. Anti-oxidants and angiotensin inhibitors have shown promise as adjunctive anti-fibrotic agents in preliminary human studies, and they exemplify a genre of interventions that are likely to influence future management strategies.
- Citation: Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol 2014; 20(10): 2515-2532
- URL: https://www.wjgnet.com/1007-9327/full/v20/i10/2515.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i10.2515
Hepatic fibrosis is commonly preceded by chronic inflammation[1,2], and persistence of this inflammation has been associated with progressive hepatic fibrosis and the development of cirrhosis[3]. Chronic viral hepatitis and autoimmune hepatitis are chronic inflammatory diseases of the liver that exemplify this progression, and studies have indicated that prompt and sustained suppression of inflammatory activity by eliminating the etiological agent (virus)[4-10] or dampening the immune response (lymphocytic proliferation and infiltration)[11-15] can halt and even reverse the fibrotic process. The current treatments of chronic liver inflammation were not designed to be anti-fibrotic[9,14], but the success of these treatments in achieving this effect can be measured in prolonged survival and possibly a reduced occurrence of hepatocellular carcinoma[10,16-21].
The molecular pathways that link chronic liver inflammation with progressive hepatic fibrosis continue to be clarified, and this evolving knowledge affords opportunities to directly target the fibrotic process[22-24]. Current conventional treatments that are focused on elimination of an etiological agent or putative immune-mediated mechanism may be supplemented in the future by agents that diminish oxidative stress, dampen hepatic stellate cell activation, reduce myofibroblast proliferation, and increase degradation of the extracellular matrix[22-24]. The challenges are to characterize the disease-specific mechanisms of fibrogenesis, establish the safety and efficacy of the anti-fibrotic interventions in a timely fashion, and incorporate them into conventional treatment regimens.
Highly selective, site-specific, anti-fibrotic therapies are unlikely to replace current treatments for the chronic inflammatory liver diseases, and their eventual emergence into clinical practice is best envisioned as a supplemental therapy[22-24]. Novel anti-fibrotic treatments must have actions that are additive to the anti-inflammatory and immunosuppressive properties that are already possessed by the conventional treatments.
The objectives of this review are to describe the mechanisms by which liver inflammation can stimulate hepatic fibrosis, discuss the putative anti-fibrotic properties of the conventional drug regimens used in the treatment of the chronic inflammatory liver diseases, detail the clinical efficacy of these current regimens, and assess the prospect of ancillary anti-fibrotic therapies.
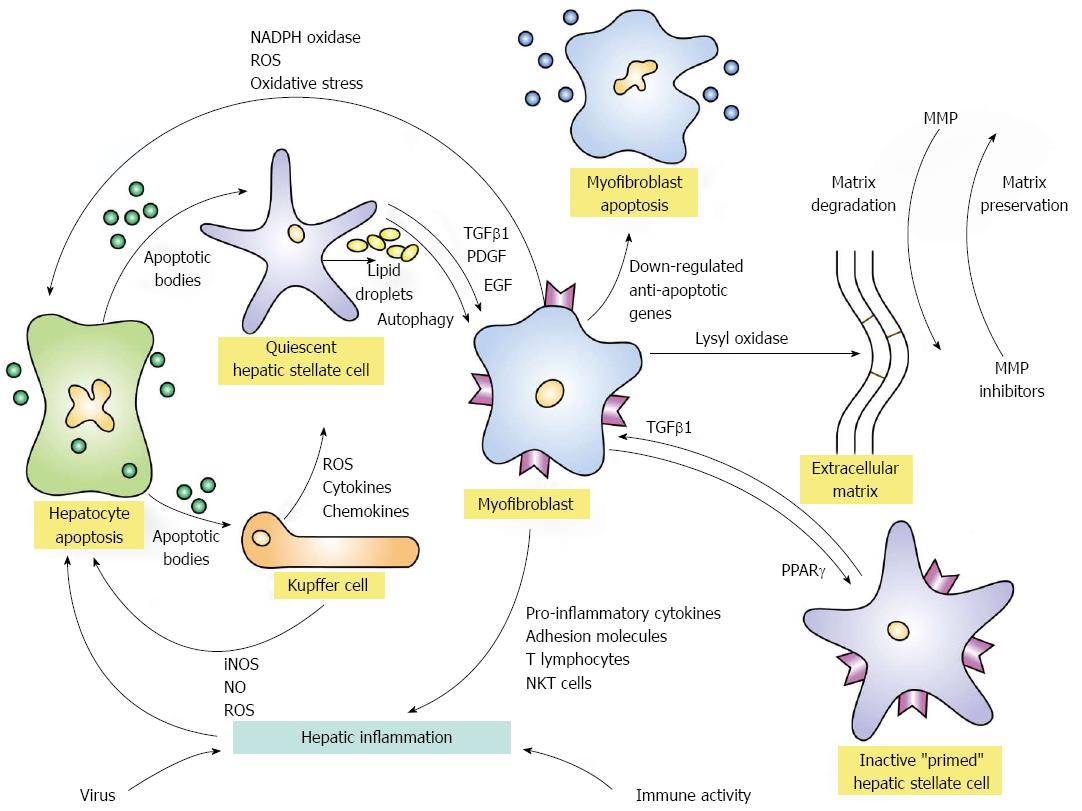
Liver inflammation is commonly associated with hepatocyte necrosis and apoptosis[1,2,23]. These forms of liver cell injury initiate a sequence of events that is independent of the etiological basis for the inflammation and can result in hepatic fibrosis. Apoptotic bodies derived from the damaged hepatocytes can activate quiescent hepatic stellate cells and Kupffer cells, and these activated cell populations can in turn promote inflammatory and fibrogenic responses[1,2,23] (Figure 1). Transforming growth factor beta 1 (TGFβ1), platelet-derived growth factor, and endothelial growth factor can induce the activated hepatic stellate cells to transform into myofibroblasts[25-33]. The activated hepatic stellate cells can also increase the production of inflammatory chemokines[34], the expression of adhesion molecules[35], and the presentation of antigens to T lymphocytes and natural killer T cells[36]. These enhanced inflammatory and immune-mediated responses can promote hepatocyte necrosis and apoptosis and thereby strengthen and perpetuate the stimuli for fibrogenesis[23,37-40].
Myofibroblasts have a contractile property that is signaled by the expression of α-smooth muscle actin[41]. They are derived from hepatic stellate cells and from portal mesenchymal cells, and their origin may reflect the nature of the liver injury and the microenvironment within the liver. The transition between hepatic stellate cells to myofibroblasts involves signaling pathways, such as Notch and Hedgehog, which modulate the epithelial-to-mesenchymal cell transition[42]. Hepatic stellate cells can be deactivated, and a mesenchymal-to-epithelial cell transition can occur which reverts the myofibroblast to an inactive hepatic stellate cell[42]. This inactive cell remains primed for reactivation, and it may be more responsive to recurrent fibrogenic stimuli than its original quiescent state[41-45]. Deactivation of the hepatic stellate cells terminates fibrogenesis and facilitates regression of the extracellular matrix[43].
The activated Kupffer cells can promote hepatic fibrogenesis by releasing cytokines and chemokines that stimulate the hepatic stellate cells[1,2,23] (Figure 1). The Kupffer cells can also release reactive oxygen species, nitric oxide, and chemotactic proteins that promote hepatocyte injury and nurture the inflammatory response. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase can stimulate the production of reactive oxygen species in hepatic stellate cells, macrophages and hepatocytes[46,47], and the resultant oxidative stress on the hepatocytes can damage deoxyribonucleic acid (DNA), induce apoptosis, promote the expression of pro-inflammatory genes, enhance fibrogenesis, and possibly trigger malignant transformation[47,48]. Inducible nitric oxide synthase (iNOS) can promote hepatocyte toxicity by increasing the production of nitric oxide, and the nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) can modulate the production of iNOS and the oxidative stress reaction[49]. The net consequence of these diverse interactive cellular and molecular mechanisms is to perpetuate and extend the tissue injury and enhance the accumulation of the extracellular matrix of collagen[50].
The overproduction and accumulation of collagens I and IV, procollagen III, and elastin occur early in liver injury, and metalloproteinases that are directed at the different types of collagen are activated to degrade the depositions and maintain stability of the matrix[23,50] (Figure 1). Tissue inhibitors of the metalloproteinases are also expressed to counter-regulate the degradation process[50,51]. They may also induce expression of the anti-apoptotic protein, Bcl-2, and thereby enhance survival of hepatic stellate cells[23,52]. Maturation of the collagen matrix depends mainly on lysyl oxidases that cross-link the collagen fibrils and increase the resistance to degradation[50,53].
Prevention or reversal of hepatic fibrosis depends on signaling pathways that influence the apoptosis of myofibroblasts, inactivation of hepatic stellate cells, and degradation of extracellular matrix (Figure 1). Myofibroblast apoptosis has been associated with the down-regulation of anti-apoptotic genes[44,45]; the peroxisome proliferator-activated receptor-gamma (PPARγ) gene has been associated with sustained quiescence of hepatic stellate cells and possibly their inactivation[45,54,55]; and metalloproteinases have degradative and fibrogenic properties that are counterbalanced[23,56,57]. Autophagy connotes the lysomal degradation of non-vital or dysfunctional cellular components, and these products can provide energy sources that maintain cell survival[58]. Hepatic stellate cells release lipid droplets (retinyl esters and triglycerides) during their activation, and this manifestation of autophagy may provide the energy necessary for their transition to myofibroblasts[59,60]. Clarification and potentiation of the signaling pathways that modulate these various actions can have therapeutic relevance.
Hepatic inflammation initiates fibrogenesis by promoting hepatocyte necrosis and apoptosis, sustains fibrogenesis by activating hepatic stellate cells and Kupffer cells, and maintains itself by the actions of pro-inflammatory cytokines and chemokines that influence the trafficking of inflammatory and immune cells within the liver[1,2] (Figure 1). The deposition of extracellular fibrillar collagen is the net result of these diverse and counter-regulated actions, and the degradation of this matrix becomes more formidable after cross-linkage of the collagen fibrils[61]. Diverse interactive and counter-regulatory mechanisms strive to maintain homeostasis, and current anti-inflammatory and immunosuppressive regimens for the chronic inflammatory liver diseases have an important, but imprecise, role in supporting this effort. Interventions that target site-specific molecular pathways implicated in the fibrotic process promise to bolster these regimens[22-24].
Anti-viral and immunosuppressive regimens have been the mainstays of therapy for the chronic inflammatory liver diseases, and the prevention or reversal of hepatic fibrosis has not been their primary objective. The dynamic nature of hepatic fibrosis had not been fully appreciated at the time of their introduction[62,63]; assessments of changes in hepatic fibrosis were hampered by sampling error and observer variation in the interpretation of liver tissue specimens[64-68]; and biomarkers more closely reflected liver inflammation than collagen deposition[69,70]. Subsequent investigations indicated that fibrosis could decrease during anti-viral therapy for chronic hepatitis C[4,9,10,71] and corticosteroid treatment for autoimmune hepatitis[11,12,14,15]. Furthermore, isolated clinical studies suggested that cirrhosis could disappear during treatment[13,14]. These experiences implicated hepatic inflammation as an important driver of liver fibrosis, and they indicated that the prevention or reversal of hepatic fibrosis was an appropriate goal of the conventional treatments for chronic inflammatory liver disease[3,14].
Treatments that eliminate the hepatitis C virus (HCV) can impair virus-induced inflammatory, immune-mediated, and fibrogenic responses (Table 1). The hepatitis C virus can activate hepatic stellate cells[72,73], and viral proteins can increase oxidative stress within hepatocytes[1,23,74] and promote the release of pro-inflammatory chemokines[75]. The introduction of non-structural proteins of HCV into hepatic stellate cells via an adenovirus vector can increase the proliferation of these cells, stimulate chemokine secretion, and enhance expression of adhesion molecules[74]. Incubation of activated human hepatic stellate cells with recombinant HCV proteins increases the production of reactive oxygen species[1,23,74], and HCV proteins also stimulate the secretion of TGFβ1 and the production of pro-collagen in cultured rat hepatic stellate cells[74].
| Conventional therapies | Possible anti-fibrotic actions | Clinical outcomes |
| Anti-viral agents | Prevent viral activation of HSC[72,74] Limit viral induction of ROS[74] Reduce HSC proliferation[72] Decrease pro-inflammatory signals[74] Reduce TGFβ1 and procollagen[72-74] Decrease lymphocyte recruitment[75] | No fibrosis at 96 wk in 68%[7] Less fibrosis after SVR in 33%[9] Fibrosis stable in non-responders[6,9] Reversal of cirrhosis possible[10] Fewer complications of cirrhosis[10] Better transplant-free survival[10] |
| Corticosteroids | Inhibit NF-κB by stimulating IκB[85] Deplete pro-inflammatory factors[86] Decrease adhesion molecules[84,87] Increase lymphocyte apoptosis[88] Reduce production of ROS[49] Suppress metalloproteinase inhibitors[90] Decrease TGFβ1 activity[91-93] Impair activation of HSC[94] | Less fibrosis in 57%[11] Reversal of cirrhosis[12,13] Less or stable fibrosis in 79%[14] Inflammation increases fibrosis[3,14] Cirrhosis survival improved[11,18,165] |
| Cyclosporine (calcineurin inhibitors) | Reduce cytokines and growth factors[162] Decrease lymphocyte proliferation[97,162] Inhibit TGFβ and interleukin-4[164] | Decreased hepatic fibrosis score[15] Reduced inflammation score[15] |
| Azathioprine | Deplete purine-based nucleotides[98-100] Impair lymphocyte proliferation[98] Increase lymphocyte apoptosis[105,106] Deplete NK cells[107] Suppress pro-inflammatory genes[108] | Conjectural anti-fibrotic effects[97] Used mainly with steroids[97,109] Possibly protective after relapse[110] |
| Mycophenolate mofetil | Inhibit lymphocyte proliferation[112,113] Increase lymphocyte apoptosis[112,113] Decrease adhesion molecules[112,113] Inhibit fibroblast proliferation[114] Reduce iNOS production[112,113] | Unproven anti-fibrotic effects[97] |
| Ursodeoxycholic acid | Limit apoptosis of hepatocytes[138] Decrease oxidative stress[139] Reduce TGFβ1 signaling in HSC[140] | Varied anti-fibrotic effects[118,120,125] Slows fibrosis progression[126] |
In patients with chronic hepatitis C, the chemokines, CCL21 and CCR7, are expressed within liver tissue, and CCL21 is concentrated around portal tracts and lymphoid nodules[75]. CD8+ T lymphocytes isolated from the liver of these patients are more commonly positive for CCR7 than cells from controls, and cultured hepatic stellate cells produce CCR7 and react to CCL21 by triggering pro-inflammatory signaling pathways that include NF-κB[75]. In this fashion, HCV can influence the intrahepatic cytokine milieu and support inflammatory and immune-mediated responses that favor fibrogenesis.
Hepatic steatosis is present in as many as 70% of patients with chronic hepatitis C[76-78]. In those patients infected with HCV genotype non-3, the frequencies of steatosis and fibrosis are higher in patients with oxidative stress[78]. Hepatic steatosis is independently associated with oxidative stress, and hepatic steatosis and the histological activity score are independent predictors of hepatic fibrosis[78]. These findings suggest that complex inter-dependent pathogenic pathways involving liver inflammation, oxidative stress, hepatic steatosis, and hepatic fibrosis are interwoven in some patients with chronic hepatitis C. Elimination of the viral agent might be the key to interrupting this fibrogenic process.
Anti-viral therapy may be anti-fibrotic because HCV infection promotes hepatic inflammation, immune-mediated responses, and signaling pathways that can enhance fibrogenesis (Table 1). HCV may also directly activate hepatic stellate cells without mediators of inflammation, and this possibility suggests a basis for the observed discrepancy between inflammatory activity and progressive hepatic fibrosis in some HCV-infected patients[72]. Other viral agents are not as well studied as triggers for fibrogenesis, but the association should be broadly accepted until proven otherwise. Elimination rather than attenuation of the viral infection should be the goal of treatment[79].
Immune-mediated liver diseases are consequences of cell-mediated and antibody-dependent mechanisms that are directed against self-antigens[80,81]. Autoimmune hepatitis lacks an etiological trigger that can be precisely targeted, and its treatment is directed in a non-selective fashion at putative inflammatory and immune-mediated mechanisms of tissue injury that can in turn promote hepatic fibrosis[82] (Table 1). Prednisolone is the active metabolite of prednisone, and it binds to a glucocorticoid receptor within the cytosol[82,83]. This complex is then translocated to the nucleus where it interacts with glucocorticoid-responsive genes and inhibits the production of pro-inflammatory cytokines[82,84].
Prednisolone also has an intracytoplasmic effect on the activity of NF-κB by stimulating the production of its inhibitor (IκB)[82,85]. Nuclear factors essential for the transcription of cytokines are depleted, and pro-inflammatory and immune-stimulatory actions are suppressed[82,86]. These actions are augmented by a prednisolone-induced reduction in the expression of adhesion molecules necessary for the targeting of inflammatory and immune cells[84,85,87]. Furthermore, prednisolone enhances the apoptosis of lymphocytes, and it can thereby interrupt an injurious immune-response[88].
Prednisolone also has broad anti-fibrotic actions (Table 1). By reducing hepatic inflammation, the signaling pathways that trigger the production of reactive oxygen species may be less active[49]. Metalloproteinase inhibitors, which are stimulated by hepatic inflammation, may be less provoked, and the degradation of fibrillar collagens by unopposed metalloproteinases may proceed more freely[89,90]. The expression of TGFβ1 may also be reduced by a glucocorticoid-responsive element in the human TGFβ1 gene promoter[91]. Furthermore, the activation and binding characteristics of TGFβ1 may be impaired by corticosteroids[92,93]. These actions may in turn reduce the transformation of hepatic stellate cells into myofibroblasts[94].
The net effect of these corticosteroid actions on the inflammatory and immune-mediated responses in autoimmune hepatitis is to limit tissue damage, reduce the signals for fibrogenesis, and restore homeostatic mechanisms that control the extracellular matrix. The multiplicity and diversity of corticosteroid actions[82,87] and the complexity and interconnectivity of the signaling pathways of fibrogenesis[1,2] limit the efficacy and consistency of corticosteroids as anti-fibrotic agents[23]. Cirrhosis is still a common consequence of autoimmune hepatitis[18,95], and corticosteroids have had variable effects on fibrogenesis in animal models[96].
Azathioprine is a purine antagonist that has anti-proliferative, pro-apoptotic, and anti-inflammatory actions that are complementary to the actions of prednisone and prednisolone, and these actions may in turn strengthen the anti-fibrotic actions of the corticosteroids[97] (Table 1). The 6-thioguanine nucleotides are the active metabolites of azathioprine, and they can impair the synthesis of purine-based nucleotides essential in the creation of new DNA and the proliferation of activated lymphocytes[98-102]. Intracellular signal transduction can also be blocked by the generation of 6-thioguanine triphosphate which in turn dampens immune cell proliferation[103]. Furthermore, the azathioprine-generated 6-thioguanine triphosphate can interrupt a dephosphorylation pathway necessary for the activation of T lymphocytes by antigen presenting cells[104]. These anti-proliferative actions can be complemented by pro-apoptotic and anti-inflammatory actions that may also impact on the signals for fibrogenesis.
Genes that regulate the expression of anti-apoptotic factors are inhibited by 6-thioguanine triphosphate, and the survival of the activated T and B lymphocytes that mediate liver injury may be shortened[105,106]. Natural killer cells that can contribute to an antibody-dependent cell-mediated liver injury may also be depleted[107]. These actions can reduce immune-mediated liver injury and secondarily, the inflammatory response to tissue damage. The 6-thioguanine nucleotides can also directly impair the inflammatory response by dampening the expression of pro-inflammatory genes[108].
The anti-fibrotic actions of azathioprine are conjectural and based on the putative actions of its active metabolites[97] and its association with the clinical findings of reduced fibrosis in patients with corticosteroid-treated autoimmune hepatitis[14]. The preferred treatment of autoimmune hepatitis is prednisone or prednisolone in combination with azathioprine, and the anti-fibrotic contributions of azathioprine to the clinical experiences with corticosteroids can only be surmised[109]. Azathioprine (2 mg/kg daily) has been used as a long-term maintenance therapy in patients with autoimmune hepatitis who have relapsed after corticosteroid withdrawal, but its anti-fibrotic effects during such treatment have not been studied[110]. The stable quiescence of the disease during maintenance therapy with azathioprine suggests that the drug may prevent progressive fibrosis by preventing exacerbations of inflammatory activity[110,111].
Mycophenolate mofetil is a next generation purine antagonist that has a different metabolic pathway than azathioprine but similar anti-proliferative and anti-inflammatory actions[97,112,113] (Table 1). The synthesis of purine-based nucleotides is impaired by mycophenolic acid, which is the active metabolite of the drug, and cell proliferation is reduced by reversible, non-competitive inhibition of inosine monophosphate dehydrogenase, the enzyme necessary for conversion of inosine monophosphate to guanosine monophosphate. Deficiencies in guanosine monophosphate can in turn dampen cell-mediated immune responses and antibody production[97,112,113]. Furthermore, mycophenolic acid can induce apoptosis of activated lymphocytes, suppress the expression of adhesion molecules, decrease the proliferation of fibroblasts, and impair the production of iNOS in macrophages[97,112-114]. By these mechanisms, mycophenolate mofetil can limit the survival of activated lymphocytes, decrease inflammatory activity, and reduce tissue damage mediated through nitric oxide production. The theoretical net effects of these actions would be to reduce tissue damage and fibrogenesis while favoring fibrinolysis by de-repressing metalloproteinases[114]. As with azathioprine, the anti-fibrotic effects of mycophenolate mofetil are unproven and not the primary objectives of treatment with this agent[97].
Ursodeoxycholic acid alone or in combination with corticosteroids has been an effective frontline therapy for autoimmune hepatitis in Japan[115-117] (Table 1). In other countries, it has been used mainly in diverse cholestatic liver diseases as the sole drug[118-120] or in syndromes with mixed features of autoimmune hepatitis and cholestasis (“overlap syndromes”) in conjunction with corticosteroids[121-123]. Unlike the drugs used for chronic viral hepatitis or autoimmune hepatitis, the principal actions of ursodeoxycholic acid are not directed at reducing liver inflammation by either eliminating an etiological agent or interrupting immune-mediated pathways[124].
Experiences reporting stabilization of histological stage in treated patients with primary biliary cirrhosis (PBC)[120] have been countered by experiences reporting no or uncertain effects of the drug on hepatic fibrosis[118,125]. Treatment of PBC with ursodeoxycholic acid has been associated with a five-fold slower rate of progression from early stage to advanced stage disease compared to untreated patients[126], and the drug probably has an anti-fibrotic effect that is manifested by the slower progression of fibrosis. The impact of ursodeoxycholic acid on hepatic fibrosis takes years to recognize, and the treatment has not been associated with regression[126].
Importantly, hepatic inflammation, manifested as lymphocytic piecemeal necrosis, is an independent predictor of progressive hepatic fibrosis in PBC[127,128], and patients with PBC and inflammatory manifestations that resemble those of autoimmune hepatitis respond poorly to PBC-directed therapies[129]. They have higher frequencies of esophageal varices, gastrointestinal bleeding, ascites, and death from liver failure or requirement for liver transplantation than patients with classical PBC[129,130], and the presence of these inflammatory manifestations has justified treatment regimens that combine corticosteroids with ursodeoxycholic acid[121,131,132]. In PBC as in chronic hepatitis C and autoimmune hepatitis, hepatic inflammation is a driver of hepatic fibrosis.
Ursodeoxycholic acid has cytoprotective, bile stimulatory, and anti-apoptotic properties in chronic cholestatic liver disease, and its anti-inflammatory or anti-fibrotic effects are consequences of these three basic properties[124,133]. Phospholipids in mixed micelles protect cholangiocytic membranes against damage by hydrophobic bile acids[134], and ursodeoxycholic acid can modulate the composition of micelles to favor the phospholipid component[135,136]. The cytoprotective actions by this hydrophilic bile acid can in turn reduce or prevent cholangiocyte injury, portal inflammation, and the generation of fibrogenic stimuli. Ursodeoxycholic acid also stimulates biliary secretion of potentially toxic hydrophobic bile acids[137], and it can thereby protect against the apoptosis of liver cells whose death receptors would otherwise be directly stimulated by increasing intracellular concentrations of the toxic bile acids[138].
Ursodeoxycholic acid has had anti-fibrotic effects in a bile duct-ligated animal model[139], and it has been found to reduce the expressions of TGFβ1, TGF type 1 receptor and other components of the signaling pathway involved in the activation of cultured rat hepatic stellate cells[140]. Furthermore, ursodeoxycholic acid may reduce fibrogenesis through a pathway involved in the synthesis of glutathione which in turn may decrease oxidative stress and the activation of hepatic stellate cells[139]. These properties have had limited effects on hepatic fibrosis in humans with chronic cholestatic liver disease[118,120,125,126], and norursodeoxycholic acid, which is a homologue of ursodeoxycholic acid, may have more potent anti-inflammatory and anti-fibrotic actions[134,141].
Norursodeoxycholic acid has a shortened side chain which distinguishes it from ursodeoxycholic acid, and it is resistant to conjugation (amidation) with taurine or glycine[141-143]. The unconjugated state and its shortened side chain renders the molecule more easily reabsorbed from bile and more rapidly re-secreted by hepatocytes[141,144]. This shunting within the enterohepatic circulation is associated with a hypercholeresis and decreased cholangitis and fibrosis in a murine model of primary sclerosing cholangitis[141]. Biliary fibrosis in murine models of chronic cholestatic liver disease correlate with the amount of ductular reaction[145-147], and norursodeoxycholic acid may attenuate this trigger for fibrosis by reducing ductular proliferation[141]. Clinical trials are needed to determine the efficacy and safety of this intervention.
The ability of conventional therapies to prevent or reverse hepatic fibrosis has been difficult to assess reliably because treatment regimens and follow-up schedules have varied and the methods for evaluating changes in hepatic fibrosis have been flawed. The sampling variations and interpretative inconsistencies of liver tissue examinations[23,64,66,148,149] have stimulated the quest for biomarkers[128,150-152] and imaging tests of hepatic fibrosis[151,153,154]. Liver tissue examination by needle biopsy, however, has remained the standard assessment of liver fibrosis as other modalities have been inaccurate, costly, or premature[152]. Furthermore, confidence in the liver tissue examination has been improved by the use of codified evaluation protocols for histological interpretation[155,156].
In chronic hepatitis C, the METAVIR scoring system developed by the French Cooperative Study Group has been the preferred scoring system[65,68,155], and in autoimmune hepatitis, the Ishak scoring system[3,14,156], which is a refinement of the earlier Knodell scoring system[157], has been preferred. Both systems are subject to sampling error and inter- and intra-observer variations[67,68]; each system can underestimate the grade and stage of the liver disease in small tissue samples[158]; and each system can anticipate a maximum staging accuracy of only 75% in tissue specimens that are ≥ 25 mm in length[68,148,159].
Importantly, the general availability of the needle biopsy assessment, the opportunity to acquire additional histological information about the disease and its response to treatment[148,160], the high concordance of the histological interpretations among pathologists (83%-84%)[67,68], and the strong association of the tissue findings with clinical outcomes[161] have counterbalanced the deficiencies intrinsic to the needle biopsy procedure. As a result, needle biopsy of liver tissue remains the principal basis for understanding the dynamics of hepatic fibrosis in patients with chronic hepatitis.
Pooled data from three randomized clinical trials involving 1509 patients with chronic hepatitis C demonstrated that patients who were treated with interferon alfa-2b in combination with ribavirin or interferon alfa-2b in combination with placebo for 48 wk had little or no hepatic fibrosis by METAVIR criteria at 96 wk more commonly than patients receiving the same regimens for 24 wk (68% vs 42% and 64% vs 24%, respectively)[7] (Table 1). The frequency of a sustained virological response, the duration of anti-viral therapy, and the histological stage of fibrosis prior to treatment were associated with the ability to prevent progressive hepatic fibrosis[7]. The efficacy of this treatment in limiting fibrosis was 68%.
Similar findings were reported in another study in which 99 patients with chronic hepatitis C were treated with interferon and ribavirin and 64 patients were treated with interferon alone[9] (Table 1). Progression of hepatic fibrosis, as assessed by changes in the METAVIR score and a semi-quantitative fibrosis score, was slowed more commonly in patients with advanced hepatic fibrosis who achieved a sustained virological response than in the non-responders[9]. Patients with cirrhosis at presentation who achieved a sustained virological response decreased their fibrosis score more commonly than non-responders (33% vs 9%), albeit fibrosis scores did not decrease by more than 2 points in any patient with cirrhosis. Importantly, the non-responders had no progression of hepatic fibrosis after 12 mo of therapy, and the anti-viral treatment may have had a protective effect even in the absence of a virological response[9], as had been reported in an earlier study[6].
The anti-fibrotic effects of anti-viral therapy in patients with chronic hepatitis C and cirrhosis have also been associated with fewer liver-related morbidities and better survival than in patients with unimproved hepatic fibrosis (Table 1). Of 96 patients with chronic hepatitis C and cirrhosis who received anti-viral therapy, 18 patients (19%) improved from stage 4 to stage 2 by the METAVIR scoring system after a median follow-up of 118 mo. Ten patients improved to stage 2; 7 patients improved to stage 1, and one patient improved to stage 0[10]. Improvements in the stage of fibrosis were associated with a reduction in the incidence of cirrhosis-related complications (ascites, hepatic encephalopathy, variceal bleeding, bacterial peritonitis, hepatocellular carcinoma, and liver transplantation) from 4 per 100 patient-years in individuals without regression of fibrosis to 0 per 100 patient-years in individuals with regression of fibrosis. Furthermore, the frequency of transplant-free survival at 10 years was higher in the patients in whom the fibrosis regressed (100% vs 74%)[10].
The composite experiences with anti-viral therapy in chronic hepatitis C confirm an anti-fibrotic effect, especially after sustained clearance of the virus, but the results are unpredictable, incomplete, and most often of low magnitude. Nevertheless, improvements in the fibrosis score have been associated with less morbidity and better survival in patients with chronic hepatitis C.
Corticosteroid therapy has improved hepatic fibrosis scores by a semi-quantitative scoring system in 57% of patients with autoimmune hepatitis who underwent paired needle biopsy examinations during a median follow-up of 49 mo, and the 10-year survival of all patients was similar to that of matched controls (90% vs 92%)[11] (Table 1). Another study indicated the loss of fibrosis and the reversal of cirrhosis (improvement in the median fibrosis score from 3.3 to 0.8 by Knodell scoring criteria) in 8 patients with autoimmune hepatitis and cirrhosis who responded to corticosteroid therapy[12]. Skepticism about the reversal of cirrhosis in needle biopsy specimens was somewhat allayed by the disappearance of cirrhosis in paired liver samples obtained by wedge biopsy in one treated patient after 14 years[13].
A larger study involving 325 liver specimens obtained by needle biopsy from 87 corticosteroid-treated patients with autoimmune hepatitis showed a reduction in the Ishak fibrosis score from 3.4 to 2.6 during a mean observation period of 63 mo[14]. Fibrosis scores improved in 53% of patients during a mean interval of 57 mo, and they did not worsen in 26% during a mean observation interval of 62 mo. In this study, corticosteroid treatment improved fibrosis or prevented its progression in 79% of patients, and the fibrosis score improved more commonly in individuals with reduced scores that reflected histological inflammation (61% vs 32%)[14].
The calcineurin inhibitors (cyclosporine and tacrolimus) can impair the activation of nuclear factors necessary for the transcription of cytokines and growth factors important in the proliferation of lymphocytes[97,162,163] (Table 1). These anti-proliferative actions can in turn reduce immune-mediated tissue injury and the recruitment of inflammatory cells to the sites of damage. Reduced production of TGFβ and interleukin-2 can have direct anti-fibrotic effects[164]. In 19 patients with autoimmune hepatitis who were treated with either cyclosporine (7 patients) or prednisolone (12 patients), mean fibrosis scores by the Ishak scoring system decreased from 4.5 to 2.2 during a mean interval of 3.4 years[15]. Reductions in the fibrosis scores were associated mainly with the use of cyclosporine and the duration of treatment, and the use of cyclosporine was the principal anti-fibrotic factor by logistic regression analysis (albeit the sample size was small and the confidence interval wide)[15]. Patients in whom fibrosis scores improved also had greater reductions in the scores for liver inflammation, and these findings re-confirmed the association between liver fibrosis and hepatic inflammation.
The composite experiences with immunosuppressive therapy in autoimmune hepatitis, including regimens based on the administration of cyclosporine, have indicated an anti-fibrotic effect in 57%-79%, and this improvement had been associated with reductions in hepatic inflammation. Reversal of cirrhosis is possible, but a limited reduction in the fibrosis score is more common.
Conventional treatments for chronic liver disease can improve hepatic fibrosis scores and increase survival, but these improvements are unpredictable, slow, and typically small. Cirrhosis in autoimmune hepatitis is uncommon during the first year of treatment (7%), but its occurrence increases to 39% at two years and 59% at 3 years if inflammatory activity continues[165]. The failure to induce resolution of hepatic inflammation within 36 mo is associated with higher frequencies of cirrhosis (54% vs 18%) and need for liver transplantation (15% vs 2%) than resolutions that occur within 12 mo[166]. In patients satisfying criteria for remission, the mean annual incidence of cirrhosis is 2.6%[165], and the risk of cirrhosis in autoimmune hepatitis persists indefinitely as a consequence of unsuspected residual or recurrent mild chronic inflammation[167,168].
The development of cirrhosis is also slow in chronic hepatitis C, but there is greater individual variability in this propensity than in autoimmune hepatitis depending on the age at the time of infection, daily alcohol consumption, and gender[169]. The median duration from infection to cirrhosis in untreated patients is 30 years, ranging from 13 years in men infected after the age of 40 years to 42 years in non-alcoholic women. Importantly, 31% of patients never develop cirrhosis or remain free of cirrhosis for at least 50 years[169]. These individual variations in the time to cirrhosis make assessments of the anti-fibrotic actions of anti-viral therapy difficult, but delays in clearing the virus or tolerating the medication probably contribute to disease progression[23].
Anti-fibrotic therapies have the potential to protect the liver during the protracted process of suppressing liver inflammation in autoimmune hepatitis and eliminating the etiological agent in chronic viral hepatitis (Table 2). Most anti-fibrotic therapies have theoretical value, limited laboratory evidence, and minimal or no human experience[22-24]. The most promising anti-fibrotic therapies that have been evaluated in humans with chronic liver disease have been the anti-oxidants[22,23]. The angiotensin inhibitor (losartin) has also had success in a limited non-randomized study[170]. Human trials of interventions that disrupt pro-inflammatory cytokine pathways mediated by tumor necrosis factor-α (infliximab[171-174], etanercept[175,176], and pentoxyphylline[177]), neurochemicals that block the fibrogenic activity of myofibroblasts (cannabinoid antagonists)[23,178], compounds that enhance the expression of nuclear receptors within hepatic stellate cells and preserve their quiescence (farglitazar)[179], and drugs that inhibit fibrogenesis by inactivating hepatic stellate cells, impairing TGFβ1 secretion, and protecting liver cells by increasing glutathione production and reducing oxidative stress (oltipraz)[180-182] have been ineffective or toxic.
| Anti-fibrotic agent | Possible anti-fibrotic actions | Clinical experiences |
| Anti-oxidants | ||
| -acetylcysteine | Inhibits NF-κB activity[195]Limits pro-inflammatory genes[195]Reduces iNOS and NO activity[196]Decreases hepatocyte apoptosis[195] | Reduced hepatic fibrosis and inflammation in NASH[191] |
| S-adenosyl-L- methionine (SAMe) | Increase mitochondrial glutathione[197]Inhibit NF-κB activity[197]Reduce ROS production[197]Impair iNOS and NO production[197]Limit HSC activation[197] | Decreased mortality and LT in alcoholic cirrhosis[190]Hastened decline of viral load and increased early response in HCV non-responders[192] |
| Vitamin E | Reduce TGF-β in animals and humans[199,200]Decrease oxidant stress on hepatocytes[198]Limit collagen deposition[198] | Decreased hepatic fibrosis in NAFLD[201]Prevented progressive hepatic fibrosis in NAFLD[202] |
| Angiotensin inhibitors | ||
| Losartin | Limit angiotensin II production by HSC[208]Decrease expression of pro-fibrotic genes[170]Limit NADPH-oxidase and oxidative stress[170]Reduce TGF-β and pro-collagen production[214]Decrease extracellular matrix[210,212,213] | Small trial in chronic hepatitis C[170]Impeded pro-fibrotic and NADPH oxidase genes[170]Reduced oxidative stress[170]Decreased inflammatory and fibrosis scores in 50%[170] |
Oxidative stress is present in 61% of patients with chronic hepatitis C irrespective of histological activity index, viral load or viral genotype as assessed by immunoglobulin G antibodies against lipid peroxidation-derived antigens (malondialdehyde adducts to human serum albumin)[78]. Immunohistochemical staining of liver tissue using monoclonal antibodies against mouse macrophage iNOS and nitrotyrosine, which reflects nitric oxide production during inflammation, has indicated oxidative stress in all specimens from patients with primary biliary cirrhosis (14 patients) and autoimmune hepatitis (10 patients)[49]. The frequency of oxidative stress in chronic liver disease and the deleterious effects of reactive oxygen species on hepatocytes have supported the use of anti-oxidants as supplemental therapies, and studies evaluating these agents in animal models[183-188], cell lines[183,189], and humans with diverse liver diseases[78,190-192] have strengthened this role. The ability of anti-oxidants to reduce liver inflammation and disease severity has also advanced their promise as anti-fibrotic agents[193,194].
N-acetylcysteine is a sulfhydril donor that inhibits the transcription activities of NF-κB, and it can reduce the expression of pro-inflammatory genes[195], modulate the expression of iNOS[183,196], and limit apoptosis by reducing nitric oxide production (Table 2)[195]. Therapy with N-acetylcysteine in combination with metformin for 12 mo has improved histological activity scores and reduced hepatic fibrosis in patients with non-alcoholic steatohepatitis[191].
SAMe can replenish mitochondria with glutathione, inhibit NF-κB activity, reduce the generation of reactive oxygen species, and limit hepatic stellate cell activation by impairing the production of iNOS and the hepatic synthesis of nitric oxide (Table 2)[197]. Therapy with SAMe has decreased mortality or the need for liver transplantation in patients with alcoholic cirrhosis from 29% to 12%, and it has improved their two-year survival[190]. Therapy with SAMe (1600 mg daily for 2 wk) has also hastened the decline in viral load and increased the frequency of an early virological response (53% vs 0%) in non-responders with chronic hepatitis C (genotype 1)[192].
Vitamin E is an anti-oxidant that protects against toxic liver injury in animals[198] and prevents hepatic fibrosis in animal models and humans with acute and chronic liver damage (Table 2). Vitamin E reduces the production of TGFβ[199,200], and it in turn impairs the activation of hepatic stellate cells[199]. It has already been shown to improve[201] or stabilize[202] hepatic fibrosis scores in non-alcoholic fatty liver disease. Folate, melatonin, taurine, and salsalate are other anti-oxidants that are candidates for study in chronic liver disease[203]. Initial interest in betaine, as a method of increasing hepatic SAMe levels and reducing hepatic steatosis[204], in alcoholic[205] and non-alcoholic liver disease[206] has waned after performance of a controlled clinical trial[207]. Anti-oxidants have not been used in autoimmune hepatitis, but the results of their use in patients with alcoholic cirrhosis, non-alcoholic steatohepatitis, and chronic hepatitis C compel their consideration.
Components of the renin-angiotensin system are expressed in multiple organs, including the heart, kidney, gonads, pituitary, adrenal glands, and liver[208,209], and angiotensin II, which is the principal product of this system, can be synthesized by activated hepatic stellate cells[208]. Locally produced angiotensin II from myofibroblasts is involved in the healing response to tissue injury, and it can induce the secretion of pro-inflammatory cytokines and the synthesis of extracellular matrix as well as inhibit collagen degradation[170,210-212]. The fibrogenic properties of angiotensin II are consequences of reactive oxygen species that are generated within hepatic stellate cells by NADPH oxidase, and interventions that disrupt the renin-angiotensin system reduce experimental hepatic fibrosis and oxidative stress[213,214].
Losartan, an angiotensin receptor antagonist, has been assessed as an anti-fibrotic agent in a small clinical trial (Table 2). Fourteen patients with chronic hepatitis C were treated for 18 mo with losartin (50 mg daily)[170]. Inflammatory activity and fibrosis stage by the METAVIR scoring system decreased in 7 patients, and the expression of profibrotic genes and genes affecting NADPH oxidase activity and oxidative stress were also reduced[170]. Viral load, serum liver tests, collagen content, and fibrosis stage were unchanged overall, but the encouraging results in 7 patients justified a recommendation for a randomized clinical trial[170].
Hepatic fibrosis can be prevented or reversed by eliminating the etiologic agent or disrupting the pathogenic mechanisms of liver injury. Studies in animal models of bile duct ligation[215] and schistosomiasis[216] and clinical experiences in patients with chronic bile duct obstruction[217], hemochromatosis[218], Wilson disease[219], jejuno-ileal bypass[220], thalessemia[221], primary biliary cirrhosis[222], chronic viral hepatitis[4-10], and autoimmune hepatitis[11,12,14,15] attest to this possibility[223]. Hepatic inflammation is only one driver of hepatic fibrosis, but it is a injurious process that can be measured by laboratory and histological indices and targeted by conventional therapies[3].
Current management strategies for the chronic inflammatory liver diseases have not been optimized to prevent or reverse hepatic fibrosis, and their potential salutary effect on this response to tissue injury has often been underestimated, unrecognized, or ignored[18,165,224]. Treatments of chronic viral hepatitis and autoimmune hepatitis are typically protracted[225-229], and advanced fibrosis and cirrhosis commonly develop late in the clinical course[23,165,169]. In autoimmune hepatitis, cirrhosis can emerge years after the presentation[165,167,168].
Conventional treatment strategies must be modified to focus on the prevention of hepatic fibrosis, and these modifications must ensure rapid viral clearance[166,226] and quick complete suppression of liver inflammation[230-233]. Furthermore, the resolutions must be durable[111]. The achievement of these objectives require early identification of the slow- or non-responder, reliable assessment of the tissue response, individualized adjustments in the treatment regimens, and the early incorporation of supplemental anti-fibrotic interventions of proven efficacy. Indefinite continuous therapy may be required in some patients[234]. Furthermore, anti-fibrotic agents are feasible as adjunctive therapies, and the identification and characterization of the preferred agent may be disease-dependent[235]. Randomized clinical trials are warranted to assess these issues, and they are only possible through a collaborative network of clinical investigators that is supported by a societal commitment to fund these studies[236].
P- Reviewers: Lakatos PL, Tanaka T, Stefanescu H S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
| 1. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1928] [Cited by in RCA: 2126] [Article Influence: 125.1] [Reference Citation Analysis (0)] |
| 2. | Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195-206. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 618] [Cited by in RCA: 717] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 3. | Czaja AJ, Carpenter HA. Progressive fibrosis during corticosteroid therapy of autoimmune hepatitis. Hepatology. 2004;39:1631-1638. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Dufour JF, DeLellis R, Kaplan MM. Regression of hepatic fibrosis in hepatitis C with long-term interferon treatment. Dig Dis Sci. 1998;43:2573-2576. [PubMed] [Cited in This Article: ] |
| 5. | Lau DT, Kleiner DE, Park Y, Di Bisceglie AM, Hoofnagle JH. Resolution of chronic delta hepatitis after 12 years of interferon alfa therapy. Gastroenterology. 1999;117:1229-1233. [PubMed] [Cited in This Article: ] |
| 6. | Sobesky R, Mathurin P, Charlotte F, Moussalli J, Olivi M, Vidaud M, Ratziu V, Opolon P, Poynard T. Modeling the impact of interferon alfa treatment on liver fibrosis progression in chronic hepatitis C: a dynamic view. The Multivirc Group. Gastroenterology. 1999;116:378-386. [PubMed] [Cited in This Article: ] |
| 7. | Poynard T, McHutchison J, Davis GL, Esteban-Mur R, Goodman Z, Bedossa P, Albrecht J. Impact of interferon alfa-2b and ribavirin on progression of liver fibrosis in patients with chronic hepatitis C. Hepatology. 2000;32:1131-1137. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in RCA: 221] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Kweon YO, Goodman ZD, Dienstag JL, Schiff ER, Brown NA, Burchardt E, Schoonhoven R, Brenner DA, Fried MW. Decreasing fibrogenesis: an immunohistochemical study of paired liver biopsies following lamivudine therapy for chronic hepatitis B. J Hepatol. 2001;35:749-755. [PubMed] [Cited in This Article: ] |
| 9. | Abergel A, Darcha C, Chevallier M, Ughetto S, Henquell C, Pol S, de Ledinghen V, Canva V, Bronowicki JP, Tran A. Histological response in patients treated by interferon plus ribavirin for hepatitis C virus-related severe fibrosis. Eur J Gastroenterol Hepatol. 2004;16:1219-1227. [PubMed] [Cited in This Article: ] |
| 10. | Mallet V, Gilgenkrantz H, Serpaggi J, Verkarre V, Vallet-Pichard A, Fontaine H, Pol S. Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med. 2008;149:399-403. [PubMed] [Cited in This Article: ] |
| 11. | Schvarcz R, Glaumann H, Weiland O. Survival and histological resolution of fibrosis in patients with autoimmune chronic active hepatitis. J Hepatol. 1993;18:15-23. [PubMed] [Cited in This Article: ] |
| 12. | Dufour JF, DeLellis R, Kaplan MM. Reversibility of hepatic fibrosis in autoimmune hepatitis. Ann Intern Med. 1997;127:981-985. [PubMed] [Cited in This Article: ] |
| 13. | Cotler SJ, Jakate S, Jensen DM. Resolution of cirrhosis in autoimmune hepatitis with corticosteroid therapy. J Clin Gastroenterol. 2001;32:428-430. [PubMed] [Cited in This Article: ] |
| 14. | Czaja AJ, Carpenter HA. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol. 2004;40:646-652. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Mohamadnejad M, Malekzadeh R, Nasseri-Moghaddam S, Hagh-Azali S, Rakhshani N, Tavangar SM, Sedaghat M, Alimohamadi SM. Impact of immunosuppressive treatment on liver fibrosis in autoimmune hepatitis. Dig Dis Sci. 2005;50:547-551. [PubMed] [Cited in This Article: ] |
| 16. | Wang KK, Czaja AJ. Hepatocellular carcinoma in corticosteroid-treated severe autoimmune chronic active hepatitis. Hepatology. 1988;8:1679-1683. [PubMed] [Cited in This Article: ] |
| 17. | Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, Shiomi S, Seki S, Kobayashi K, Otani S. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051-1055. [PubMed] [Cited in This Article: ] |
| 18. | Roberts SK, Therneau TM, Czaja AJ. Prognosis of histological cirrhosis in type 1 autoimmune hepatitis. Gastroenterology. 1996;110:848-857. [PubMed] [Cited in This Article: ] |
| 19. | Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280-288, 288.e1. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in RCA: 258] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 20. | Lok AS, Everhart JE, Wright EC, Di Bisceglie AM, Kim HY, Sterling RK, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis C. Gastroenterology. 2011;140:840-849; quiz e12. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 21. | Bruix J, Poynard T, Colombo M, Schiff E, Burak K, Heathcote EJ, Berg T, Poo JL, Mello CB, Guenther R. Maintenance therapy with peginterferon alfa-2b does not prevent hepatocellular carcinoma in cirrhotic patients with chronic hepatitis C. Gastroenterology. 2011;140:1990-1999. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Ghiassi-Nejad Z, Friedman SL. Advances in antifibrotic therapy. Expert Rev Gastroenterol Hepatol. 2008;2:803-816. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Cohen-Naftaly M, Friedman SL. Current status of novel antifibrotic therapies in patients with chronic liver disease. Therap Adv Gastroenterol. 2011;4:391-417. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | Ahmad A, Ahmad R. Understanding the mechanism of hepatic fibrosis and potential therapeutic approaches. Saudi J Gastroenterol. 2012;18:155-167. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Friedman SL, Arthur MJ. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989;84:1780-1785. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in RCA: 301] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Bachem MG, Melchior R, Gressner AM. The role of thrombocytes in liver fibrogenesis: effects of platelet lysate and thrombocyte-derived growth factors on the mitogenic activity and glycosaminoglycan synthesis of cultured rat liver fat storing cells. J Clin Chem Clin Biochem. 1989;27:555-565. [PubMed] [Cited in This Article: ] |
| 27. | Czaja MJ, Weiner FR, Flanders KC, Giambrone MA, Wind R, Biempica L, Zern MA. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J Cell Biol. 1989;108:2477-2482. [PubMed] [Cited in This Article: ] |
| 28. | Wong L, Yamasaki G, Johnson RJ, Friedman SL. Induction of beta-platelet-derived growth factor receptor in rat hepatic lipocytes during cellular activation in vivo and in culture. J Clin Invest. 1994;94:1563-1569. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in RCA: 241] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Guyot C, Lepreux S, Combe C, Doudnikoff E, Bioulac-Sage P, Balabaud C, Desmoulière A. Hepatic fibrosis and cirrhosis: the (myo)fibroblastic cell subpopulations involved. Int J Biochem Cell Biol. 2006;38:135-151. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Breitkopf K, Godoy P, Ciuclan L, Singer MV, Dooley S. TGF-beta/Smad signaling in the injured liver. Z Gastroenterol. 2006;44:57-66. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 31. | Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284-292. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in RCA: 394] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 32. | Hayashi H, Sakai T. Biological Significance of Local TGF-β Activation in Liver Diseases. Front Physiol. 2012;3:12. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Presser LD, McRae S, Waris G. Activation of TGF-β1 promoter by hepatitis C virus-induced AP-1 and Sp1: role of TGF-β1 in hepatic stellate cell activation and invasion. PLoS One. 2013;8:e56367. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Schwabe RF, Bataller R, Brenner DA. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am J Physiol Gastrointest Liver Physiol. 2003;285:G949-G958. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 35. | Hellerbrand SC, Tsukamoto H, Brenner DA, Rippe RA. Expression of intracellular adhesion molecule 1 by activated hepatic stellate cells. Hepatology. 1996;24:670-676. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in RCA: 116] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SH. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117-129. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in RCA: 302] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 37. | Safadi R, Ohta M, Alvarez CE, Fiel MI, Bansal M, Mehal WZ, Friedman SL. Immune stimulation of hepatic fibrogenesis by CD8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology. 2004;127:870-882. [PubMed] [Cited in This Article: ] |
| 38. | Imamura M, Ogawa T, Sasaguri Y, Chayama K, Ueno H. Suppression of macrophage infiltration inhibits activation of hepatic stellate cells and liver fibrogenesis in rats. Gastroenterology. 2005;128:138-146. [PubMed] [Cited in This Article: ] |
| 39. | Tanaka H, Leung PS, Kenny TP, Gershwin ME, Bowlus CL. Immunological orchestration of liver fibrosis. Clin Rev Allergy Immunol. 2012;43:220-229. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Hintermann E, Ehser J, Bayer M, Pfeilschifter JM, Christen U. Mechanism of autoimmune hepatic fibrogenesis induced by an adenovirus encoding the human liver autoantigen cytochrome P450 2D6. J Autoimmun. 2013;44:49-60. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Lemoinne S, Cadoret A, El Mourabit H, Thabut D, Housset C. Origins and functions of liver myofibroblasts. Biochim Biophys Acta. 2013;1832:948-954. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 42. | Xie G, Karaca G, Swiderska-Syn M, Michelotti GA, Kruger L, Chen Y, Premont RT, Choi SS, Diehl AM. Cross-talk between Notch and Hedgehog regulates hepatic stellate cell fate in mice. Hepatology. 2013;Epub ahead of print. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Troeger JS, Mederacke I, Gwak GY, Dapito DH, Mu X, Hsu CC, Pradere JP, Friedman RA, Schwabe RF. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143:1073-1083.e22. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in RCA: 386] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 44. | Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, Moore-Morris T, Scott B, Tsukamoto H. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci USA. 2012;109:9448-9453. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 507] [Cited by in RCA: 609] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 45. | Friedman SL. Fibrogenic cell reversion underlies fibrosis regression in liver. Proc Natl Acad Sci USA. 2012;109:9230-9231. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Wheeler MD, Kono H, Yin M, Nakagami M, Uesugi T, Arteel GE, Gäbele E, Rusyn I, Yamashina S, Froh M. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med. 2001;31:1544-1549. [PubMed] [Cited in This Article: ] |
| 47. | Bhogal RH, Curbishley SM, Weston CJ, Adams DH, Afford SC. Reactive oxygen species mediate human hepatocyte injury during hypoxia/reoxygenation. Liver Transpl. 2010;16:1303-1313. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 48. | Tanaka H, Fujita N, Sugimoto R, Urawa N, Horiike S, Kobayashi Y, Iwasa M, Ma N, Kawanishi S, Watanabe S. Hepatic oxidative DNA damage is associated with increased risk for hepatocellular carcinoma in chronic hepatitis C. Br J Cancer. 2008;98:580-586. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 49. | Sanz-Cameno P, Medina J, García-Buey L, García-Sánchez A, Borque MJ, Martín-Vílchez S, Gamallo C, Jones EA, Moreno-Otero R. Enhanced intrahepatic inducible nitric oxide synthase expression and nitrotyrosine accumulation in primary biliary cirrhosis and autoimmune hepatitis. J Hepatol. 2002;37:723-729. [PubMed] [Cited in This Article: ] |
| 50. | Desmoulière A, Darby I, Costa AM, Raccurt M, Tuchweber B, Sommer P, Gabbiani G. Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab Invest. 1997;76:765-778. [PubMed] [Cited in This Article: ] |
| 51. | Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJ. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology. 1996;110:821-831. [PubMed] [Cited in This Article: ] |
| 52. | Yoshiji H, Kuriyama S, Miyamoto Y, Thorgeirsson UP, Gomez DE, Kawata M, Yoshii J, Ikenaka Y, Noguchi R, Tsujinoue H. Tissue inhibitor of metalloproteinases-1 promotes liver fibrosis development in a transgenic mouse model. Hepatology. 2000;32:1248-1254. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in RCA: 205] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 53. | Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009-1017. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 618] [Cited by in RCA: 660] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 54. | Marra F, Efsen E, Romanelli RG, Caligiuri A, Pastacaldi S, Batignani G, Bonacchi A, Caporale R, Laffi G, Pinzani M. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119:466-478. [PubMed] [Cited in This Article: ] |
| 55. | Mann J, Chu DC, Maxwell A, Oakley F, Zhu NL, Tsukamoto H, Mann DA. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology. 2010;138:705-714, 714.e1-4. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in RCA: 318] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 56. | Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245-G249. [PubMed] [Cited in This Article: ] |
| 57. | Takahara T, Furui K, Yata Y, Jin B, Zhang LP, Nambu S, Sato H, Seiki M, Watanabe A. Dual expression of matrix metalloproteinase-2 and membrane-type 1-matrix metalloproteinase in fibrotic human livers. Hepatology. 1997;26:1521-1529. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in RCA: 113] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Czaja MJ, Ding WX, Donohue TM, Friedman SL, Kim JS, Komatsu M, Lemasters JJ, Lemoine A, Lin JD, Ou JH. Functions of autophagy in normal and diseased liver. Autophagy. 2013;9:1131-1158. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in RCA: 363] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 59. | Hernández-Gea V, Ghiassi-Nejad Z, Rozenfeld R, Gordon R, Fiel MI, Yue Z, Czaja MJ, Friedman SL. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142:938-946. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 495] [Cited by in RCA: 509] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 60. | Hernández-Gea V, Friedman SL. Autophagy fuels tissue fibrogenesis. Autophagy. 2012;8:849-850. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 61. | Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-Sadler H, Gaca MD, Sands E, Suliman I, Trim N, Knorr A. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795-1808. [PubMed] [Cited in This Article: ] |
| 62. | Wanless IR, Nakashima E, Sherman M. Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med. 2000;124:1599-1607. [PubMed] [Cited in This Article: ] |
| 63. | Wanless IR. Use of corticosteroid therapy in autoimmune hepatitis resulting in the resolution of cirrhosis. J Clin Gastroenterol. 2001;32:371-372. [PubMed] [Cited in This Article: ] |
| 64. | Soloway RD, Baggenstoss AH, Schoenfield LJ, Summerskill WH. Observer error and sampling variability tested in evaluation of hepatitis and cirrhosis by liver biopsy. Am J Dig Dis. 1971;16:1082-1086. [PubMed] [Cited in This Article: ] |
| 65. | French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15-20. [PubMed] [Cited in This Article: ] |
| 66. | Theodossi A, Skene AM, Portmann B, Knill-Jones RP, Patrick RS, Tate RA, Kealey W, Jarvis KJ, O’Brian DJ, Williams R. Observer variation in assessment of liver biopsies including analysis by kappa statistics. Gastroenterology. 1980;79:232-241. [PubMed] [Cited in This Article: ] |
| 67. | Westin J, Lagging LM, Wejstål R, Norkrans G, Dhillon AP. Interobserver study of liver histopathology using the Ishak score in patients with chronic hepatitis C virus infection. Liver. 1999;19:183-187. [PubMed] [Cited in This Article: ] |
| 68. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1193] [Cited by in RCA: 1386] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 69. | McCullough AJ, Stassen WN, Wiesner RH, Czaja AJ. Serum type III procollagen peptide concentrations in severe chronic active hepatitis: relationship to cirrhosis and disease activity. Hepatology. 1987;7:49-54. [PubMed] [Cited in This Article: ] |
| 70. | McCullough AJ, Stassen WN, Wiesner RH, Czaja AJ. Serial determinations of the amino-terminal peptide of type III procollagen in severe chronic active hepatitis. J Lab Clin Med. 1987;109:55-61. [PubMed] [Cited in This Article: ] |
| 71. | Arthur MJ. Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology. 2002;122:1525-1528. [PubMed] [Cited in This Article: ] |
| 72. | Schulze-Krebs A, Preimel D, Popov Y, Bartenschlager R, Lohmann V, Pinzani M, Schuppan D. Hepatitis C virus-replicating hepatocytes induce fibrogenic activation of hepatic stellate cells. Gastroenterology. 2005;129:246-258. [PubMed] [Cited in This Article: ] |
| 73. | Shin JY, Hur W, Wang JS, Jang JW, Kim CW, Bae SH, Jang SK, Yang SH, Sung YC, Kwon OJ. HCV core protein promotes liver fibrogenesis via up-regulation of CTGF with TGF-beta1. Exp Mol Med. 2005;37:138-145. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 74. | Bataller R, Paik YH, Lindquist JN, Lemasters JJ, Brenner DA. Hepatitis C virus core and nonstructural proteins induce fibrogenic effects in hepatic stellate cells. Gastroenterology. 2004;126:529-540. [PubMed] [Cited in This Article: ] |
| 75. | Bonacchi A, Petrai I, Defranco RM, Lazzeri E, Annunziato F, Efsen E, Cosmi L, Romagnani P, Milani S, Failli P. The chemokine CCL21 modulates lymphocyte recruitment and fibrosis in chronic hepatitis C. Gastroenterology. 2003;125:1060-1076. [PubMed] [Cited in This Article: ] |
| 76. | Czaja AJ, Carpenter HA. Sensitivity, specificity, and predictability of biopsy interpretations in chronic hepatitis. Gastroenterology. 1993;105:1824-1832. [PubMed] [Cited in This Article: ] |
| 77. | Czaja AJ, Carpenter HA, Santrach PJ, Moore SB. Host- and disease-specific factors affecting steatosis in chronic hepatitis C. J Hepatol. 1998;29:198-206. [PubMed] [Cited in This Article: ] |
| 78. | Vidali M, Tripodi MF, Ivaldi A, Zampino R, Occhino G, Restivo L, Sutti S, Marrone A, Ruggiero G, Albano E. Interplay between oxidative stress and hepatic steatosis in the progression of chronic hepatitis C. J Hepatol. 2008;48:399-406. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 79. | Haque M, Yoshida EM. Hepatitis C antiviral long-term treatment against cirrhosis (HALT-C) trial. Ann Hepatol. 2009;8:78-79. [PubMed] [Cited in This Article: ] |
| 80. | Czaja AJ. Autoimmune hepatitis. Part A: pathogenesis. Expert Rev Gastroenterol Hepatol. 2007;1:113-128. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 81. | Czaja AJ, Manns MP. Advances in the diagnosis, pathogenesis, and management of autoimmune hepatitis. Gastroenterology. 2010;139:58-72.e4. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in RCA: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 82. | Czaja AJ. Drug choices in autoimmune hepatitis: part A--Steroids. Expert Rev Gastroenterol Hepatol. 2012;6:603-615. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Brattsand R, Linden M. Cytokine modulation by glucocorticoids: mechanisms and actions in cellular studies. Aliment Pharmacol Ther. 1996;10 Suppl 2:81-90; discussion 91-92. [PubMed] [Cited in This Article: ] |
| 84. | De Bosscher K, Vanden Berghe W, Haegeman G. Mechanisms of anti-inflammatory action and of immunosuppression by glucocorticoids: negative interference of activated glucocorticoid receptor with transcription factors. J Neuroimmunol. 2000;109:16-22. [PubMed] [Cited in This Article: ] |
| 85. | Almawi WY. Molecular mechanisms of glucocorticoid effects. Mod Asp Immunobiol. 2001;2:78-82. [Cited in This Article: ] |
| 86. | Almawi WY, Beyhum HN, Rahme AA, Rieder MJ. Regulation of cytokine and cytokine receptor expression by glucocorticoids. J Leukoc Biol. 1996;60:563-572. [PubMed] [Cited in This Article: ] |
| 87. | Czock D, Keller F, Rasche FM, Häussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 2005;44:61-98. [PubMed] [Cited in This Article: ] |
| 88. | Migita K, Eguchi K, Kawabe Y, Nakamura T, Shirabe S, Tsukada T, Ichinose Y, Nakamura H, Nagataki S. Apoptosis induction in human peripheral blood T lymphocytes by high-dose steroid therapy. Transplantation. 1997;63:583-587. [PubMed] [Cited in This Article: ] |
| 89. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [PubMed] [Cited in This Article: ] |
| 90. | Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538-549. [PubMed] [Cited in This Article: ] |
| 91. | Parrelli JM, Meisler N, Cutroneo KR. Identification of a glucocorticoid response element in the human transforming growth factor beta 1 gene promoter. Int J Biochem Cell Biol. 1998;30:623-627. [PubMed] [Cited in This Article: ] |
| 92. | Centrella M, McCarthy TL, Canalis E. Glucocorticoid regulation of transforming growth factor beta 1 activity and binding in osteoblast-enriched cultures from fetal rat bone. Mol Cell Biol. 1991;11:4490-4496. [PubMed] [Cited in This Article: ] |
| 93. | Shukla A, Meisler N, Cutroneo KR. Perspective article: transforming growth factor-beta: crossroad of glucocorticoid and bleomycin regulation of collagen synthesis in lung fibroblasts. Wound Repair Regen. 1999;7:133-140. [PubMed] [Cited in This Article: ] |
| 94. | Bolkenius U, Hahn D, Gressner AM, Breitkopf K, Dooley S, Wickert L. Glucocorticoids decrease the bioavailability of TGF-beta which leads to a reduced TGF-beta signaling in hepatic stellate cells. Biochem Biophys Res Commun. 2004;325:1264-1270. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 95. | Czaja AJ, Carpenter HA. Distinctive clinical phenotype and treatment outcome of type 1 autoimmune hepatitis in the elderly. Hepatology. 2006;43:532-538. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 96. | Melgert BN, Olinga P, Van Der Laan JM, Weert B, Cho J, Schuppan D, Groothuis GM, Meijer DK, Poelstra K. Targeting dexamethasone to Kupffer cells: effects on liver inflammation and fibrosis in rats. Hepatology. 2001;34:719-728. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 97. | Czaja AJ. Drug choices in autoimmune hepatitis: part B--Nonsteroids. Expert Rev Gastroenterol Hepatol. 2012;6:617-635. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Chan GL, Erdmann GR, Gruber SA, Matas AJ, Canafax DM. Azathioprine metabolism: pharmacokinetics of 6-mercaptopurine, 6-thiouric acid and 6-thioguanine nucleotides in renal transplant patients. J Clin Pharmacol. 1990;30:358-363. [PubMed] [Cited in This Article: ] |
| 99. | Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol. 1992;43:329-339. [PubMed] [Cited in This Article: ] |
| 100. | Allison AC. Immunosuppressive drugs: the first 50 years and a glance forward. Immunopharmacology. 2000;47:63-83. [PubMed] [Cited in This Article: ] |
| 101. | Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, and safety. Clin Gastroenterol Hepatol. 2004;2:731-743. [PubMed] [Cited in This Article: ] |
| 102. | Atreya I, Neurath MF. Azathioprine in inflammatory bowel disease: improved molecular insights and resulting clinical implications. Expert Rev Gastroenterol Hepatol. 2008;2:23-34. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 103. | de Boer NK, van Bodegraven AA, Jharap B, de Graaf P, Mulder CJ. Drug Insight: pharmacology and toxicity of thiopurine therapy in patients with IBD. Nat Clin Pract Gastroenterol Hepatol. 2007;4:686-694. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 104. | Poppe D, Tiede I, Fritz G, Becker C, Bartsch B, Wirtz S, Strand D, Tanaka S, Galle PR, Bustelo XR. Azathioprine suppresses ezrin-radixin-moesin-dependent T cell-APC conjugation through inhibition of Vav guanosine exchange activity on Rac proteins. J Immunol. 2006;176:640-651. [PubMed] [Cited in This Article: ] |
| 105. | Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111:1133-1145. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in RCA: 567] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 106. | Mudter J, Neurath MF. Apoptosis of T cells and the control of inflammatory bowel disease: therapeutic implications. Gut. 2007;56:293-303. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 107. | Steel AW, Mela CM, Lindsay JO, Gazzard BG, Goodier MR. Increased proportion of CD16(+) NK cells in the colonic lamina propria of inflammatory bowel disease patients, but not after azathioprine treatment. Aliment Pharmacol Ther. 2011;33:115-126. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 108. | Thomas CW, Myhre GM, Tschumper R, Sreekumar R, Jelinek D, McKean DJ, Lipsky JJ, Sandborn WJ, Egan LJ. Selective inhibition of inflammatory gene expression in activated T lymphocytes: a mechanism of immune suppression by thiopurines. J Pharmacol Exp Ther. 2005;312:537-545. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 109. | Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193-2213. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1039] [Cited by in RCA: 999] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 110. | Johnson PJ, McFarlane IG, Williams R. Azathioprine for long-term maintenance of remission in autoimmune hepatitis. N Engl J Med. 1995;333:958-963. [PubMed] [Cited in This Article: ] |
| 111. | Montano-Loza AJ, Carpenter HA, Czaja AJ. Consequences of treatment withdrawal in type 1 autoimmune hepatitis. Liver Int. 2007;27:507-515. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 112. | Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85-118. [PubMed] [Cited in This Article: ] |
| 113. | Allison AC. Mechanisms of action of mycophenolate mofetil. Lupus. 2005;14 Suppl 1:s2-s8. [PubMed] [Cited in This Article: ] |
| 114. | Morath C, Schwenger V, Beimler J, Mehrabi A, Schmidt J, Zeier M, Muranyi W. Antifibrotic actions of mycophenolic acid. Clin Transplant. 2006;20 Suppl 17:25-29. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 115. | Nakamura K, Yoneda M, Yokohama S, Tamori K, Sato Y, Aso K, Aoshima M, Hasegawa T, Makino I. Efficacy of ursodeoxycholic acid in Japanese patients with type 1 autoimmune hepatitis. J Gastroenterol Hepatol. 1998;13:490-495. [PubMed] [Cited in This Article: ] |
| 116. | Miyake Y, Iwasaki Y, Kobashi H, Yasunaka T, Ikeda F, Takaki A, Okamoto R, Takaguchi K, Ikeda H, Makino Y. Efficacy of ursodeoxycholic acid for Japanese patients with autoimmune hepatitis. Hepatol Int. 2009;3:556-562. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 117. | Abe M, Mashiba T, Zeniya M, Yamamoto K, Onji M, Tsubouchi H. Present status of autoimmune hepatitis in Japan: a nationwide survey. J Gastroenterol. 2011;46:1136-1141. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 118. | Poupon RE, Balkau B, Eschwège E, Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med. 1991;324:1548-1554. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 570] [Cited by in RCA: 539] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 119. | Abuaf N, Lunel F, Giral P, Borotto E, Laperche S, Poupon R, Opolon P, Huraux JM, Homberg JC. Non-organ specific autoantibodies associated with chronic C virus hepatitis. J Hepatol. 1993;18:359-364. [PubMed] [Cited in This Article: ] |
| 120. | Parés A, Caballería L, Rodés J, Bruguera M, Rodrigo L, García-Plaza A, Berenguer J, Rodríguez-Martínez D, Mercader J, Velicia R. Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis: results of a double-blind controlled multicentric trial. UDCA-Cooperative Group from the Spanish Association for the Study of the Liver. J Hepatol. 2000;32:561-566. [PubMed] [Cited in This Article: ] |
| 121. | Chazouillères O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28:296-301. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 506] [Cited by in RCA: 466] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 122. | Chazouillères O, Wendum D, Serfaty L, Rosmorduc O, Poupon R. Long term outcome and response to therapy of primary biliary cirrhosis-autoimmune hepatitis overlap syndrome. J Hepatol. 2006;44:400-406. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 123. | Floreani A, Rizzotto ER, Ferrara F, Carderi I, Caroli D, Blasone L, Baldo V. Clinical course and outcome of autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome. Am J Gastroenterol. 2005;100:1516-1522. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 124. | Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525-531. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in RCA: 463] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 125. | Heathcote EJ, Cauch-Dudek K, Walker V, Bailey RJ, Blendis LM, Ghent CN, Michieletti P, Minuk GY, Pappas SC, Scully LJ. The Canadian Multicenter Double-blind Randomized Controlled Trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1994;19:1149-1156. [PubMed] [Cited in This Article: ] |
| 126. | Corpechot C, Carrat F, Bonnand AM, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on liver fibrosis progression in primary biliary cirrhosis. Hepatology. 2000;32:1196-1199. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in RCA: 201] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 127. | Corpechot C, Carrat F, Poupon R, Poupon RE. Primary biliary cirrhosis: incidence and predictive factors of cirrhosis development in ursodiol-treated patients. Gastroenterology. 2002;122:652-658. [PubMed] [Cited in This Article: ] |
| 128. | Corpechot C, Poujol-Robert A, Wendum D, Galotte M, Chrétien Y, Poupon RE, Poupon R. Biochemical markers of liver fibrosis and lymphocytic piecemeal necrosis in UDCA-treated patients with primary biliary cirrhosis. Liver Int. 2004;24:187-193. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 129. | Silveira MG, Talwalkar JA, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary biliary cirrhosis: long-term outcomes. Am J Gastroenterol. 2007;102:1244-1250. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 130. | Neuhauser M, Bjornsson E, Treeprasertsuk S, Enders F, Silveira M, Talwalkar J, Lindor K. Autoimmune hepatitis-PBC overlap syndrome: a simplified scoring system may assist in the diagnosis. Am J Gastroenterol. 2010;105:345-353. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 131. | Beuers U, Boberg KM, Chapman RW, Chazouilleres O, Invernizzi P, Jones DEJ, Lammert F, Pares A, Trauner M. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1227] [Cited by in RCA: 1183] [Article Influence: 73.9] [Reference Citation Analysis (1)] |
| 132. | Boberg KM, Chapman RW, Hirschfield GM, Lohse AW, Manns MP, Schrumpf E. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54:374-385. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in RCA: 324] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 133. | Hofmann AF. Pharmacology of ursodeoxycholic acid, an enterohepatic drug. Scand J Gastroenterol Suppl. 1994;204:1-15. [PubMed] [Cited in This Article: ] |
| 134. | Trauner M, Fickert P, Halilbasic E, Moustafa T. Lessons from the toxic bile concept for the pathogenesis and treatment of cholestatic liver diseases. Wien Med Wochenschr. 2008;158:542-548. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 135. | Güldütuna S, Zimmer G, Imhof M, Bhatti S, You T, Leuschner U. Molecular aspects of membrane stabilization by ursodeoxycholate [see comment]. Gastroenterology. 1993;104:1736-1744. [PubMed] [Cited in This Article: ] |
| 136. | Heuman DM, Bajaj RS, Lin Q. Adsorption of mixtures of bile salt taurine conjugates to lecithin-cholesterol membranes: implications for bile salt toxicity and cytoprotection. J Lipid Res. 1996;37:562-573. [PubMed] [Cited in This Article: ] |
| 137. | Beuers U, Bilzer M, Chittattu A, Kullak-Ublick GA, Keppler D, Paumgartner G, Dombrowski F. Tauroursodeoxycholic acid inserts the apical conjugate export pump, Mrp2, into canalicular membranes and stimulates organic anion secretion by protein kinase C-dependent mechanisms in cholestatic rat liver. Hepatology. 2001;33:1206-1216. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in RCA: 184] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 138. | Faubion WA, Guicciardi ME, Miyoshi H, Bronk SF, Roberts PJ, Svingen PA, Kaufmann SH, Gores GJ. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103:137-145. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in RCA: 410] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 139. | Ramani K, Tomasi ML, Yang H, Ko K, Lu SC. Mechanism and significance of changes in glutamate-cysteine ligase expression during hepatic fibrogenesis. J Biol Chem. 2012;287:36341-36355. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 140. | Liang TJ, Yuan JH, Tan YR, Ren WH, Han GQ, Zhang J, Wang LC, Qin CY. Effect of ursodeoxycholic acid on TGF beta1/Smad signaling pathway in rat hepatic stellate cells. Chin Med J (Engl). 2009;122:1209-1213. [PubMed] [Cited in This Article: ] |
| 141. | Halilbasic E, Fiorotto R, Fickert P, Marschall HU, Moustafa T, Spirli C, Fuchsbichler A, Gumhold J, Silbert D, Zatloukal K. Side chain structure determines unique physiologic and therapeutic properties of norursodeoxycholic acid in Mdr2-/- mice. Hepatology. 2009;49:1972-1981. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 142. | Hofmann AF, Zakko SF, Lira M, Clerici C, Hagey LR, Lambert KK, Steinbach JH, Schteingart CD, Olinga P, Groothuis GM. Novel biotransformation and physiological properties of norursodeoxycholic acid in humans. Hepatology. 2005;42:1391-1398. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 143. | Fickert P, Wagner M, Marschall HU, Fuchsbichler A, Zollner G, Tsybrovskyy O, Zatloukal K, Liu J, Waalkes MP, Cover C. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2006;130:465-481. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 144. | Yoon YB, Hagey LR, Hofmann AF, Gurantz D, Michelotti EL, Steinbach JH. Effect of side-chain shortening on the physiologic properties of bile acids: hepatic transport and effect on biliary secretion of 23-nor-ursodeoxycholate in rodents. Gastroenterology. 1986;90:837-852. [PubMed] [Cited in This Article: ] |
| 145. | Fickert P, Stöger U, Fuchsbichler A, Moustafa T, Marschall HU, Weiglein AH, Tsybrovskyy O, Jaeschke H, Zatloukal K, Denk H. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525-536. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in RCA: 280] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 146. | Sánchez-Muñoz D, Castellano-Megías VM, Romero-Gómez M. Expression of bcl-2 in ductular proliferation is related to periportal hepatic stellate cell activation and fibrosis progression in patients with autoimmune cholestasis. Dig Liver Dis. 2007;39:262-266. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 147. | Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology. 2005;41:809-818. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 148. | Czaja AJ, Carpenter HA. Optimizing diagnosis from the medical liver biopsy. Clin Gastroenterol Hepatol. 2007;5:898-907. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 149. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017-1044. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1449] [Cited by in RCA: 1530] [Article Influence: 95.6] [Reference Citation Analysis (1)] |
| 150. | Ngo Y, Munteanu M, Messous D, Charlotte F, Imbert-Bismut F, Thabut D, Lebray P, Thibault V, Benhamou Y, Moussalli J. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887-1896. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in RCA: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 151. | Poynard T, Ngo Y, Munteanu M, Thabut D, Ratziu V. Noninvasive Markers of Hepatic Fibrosis in Chronic Hepatitis B. Curr Hepat Rep. 2011;10:87-97. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 152. | Jarcuska P, Janicko M, Veselíny E, Jarcuska P, Skladaný L. Circulating markers of liver fibrosis progression. Clin Chim Acta. 2010;411:1009-1017. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 153. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [PubMed] [Cited in This Article: ] |
| 154. | Talwalkar JA, Yin M, Fidler JL, Sanderson SO, Kamath PS, Ehman RL. Magnetic resonance imaging of hepatic fibrosis: emerging clinical applications. Hepatology. 2008;47:332-342. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in RCA: 262] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 155. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 2860] [Cited by in RCA: 3047] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 156. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [PubMed] [Cited in This Article: ] |
| 157. | Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431-435. [PubMed] [Cited in This Article: ] |
| 158. | Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239-244. [PubMed] [Cited in This Article: ] |
| 159. | Scheuer PJ. Liver biopsy size matters in chronic hepatitis: bigger is better. Hepatology. 2003;38:1356-1358. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 160. | Carpenter HA, Czaja AJ. The role of histologic evaluation in the diagnosis and management of autoimmune hepatitis and its variants. Clin Liver Dis. 2002;6:685-705. [PubMed] [Cited in This Article: ] |
| 161. | Everhart JE, Wright EC, Goodman ZD, Dienstag JL, Hoefs JC, Kleiner DE, Ghany MG, Mills AS, Nash SR, Govindarajan S. Prognostic value of Ishak fibrosis stage: findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology. 2010;51:585-594. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 162. | Canafax DM, Ascher NL. Cyclosporine immunosuppression. Clin Pharm. 1983;2:515-524. [PubMed] [Cited in This Article: ] |
| 163. | Martínez-Martínez S, Redondo JM. Inhibitors of the calcineurin/NFAT pathway. Curr Med Chem. 2004;11:997-1007. [PubMed] [Cited in This Article: ] |
| 164. | Pissaia A, Aoudjehane L, Ben Othman S, Scatton O, Soubrane O, Housset C, Calmus Y, Conti F. Cyclosporine inhibits profibrotic effects of interleukin-4 and transforming growth factor β on human intrahepatic fibroblasts cultured in vitro. Transplant Proc. 2010;42:4343-4346. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 165. | Davis GL, Czaja AJ, Ludwig J. Development and prognosis of histologic cirrhosis in corticosteroid-treated hepatitis B surface antigen-negative chronic active hepatitis. Gastroenterology. 1984;87:1222-1227. [PubMed] [Cited in This Article: ] |
| 166. | Czaja AJ. Rapidity of treatment response and outcome in type 1 autoimmune hepatitis. J Hepatol. 2009;51:161-167. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 167. | Czaja AJ. Features and consequences of untreated type 1 autoimmune hepatitis. Liver Int. 2009;29:816-823. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 168. | Czaja AJ. Late relapse of type 1 autoimmune hepatitis after corticosteroid withdrawal. Dig Dis Sci. 2010;55:1761-1769. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 169. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [PubMed] [Cited in This Article: ] |
| 170. | Colmenero J, Bataller R, Sancho-Bru P, Domínguez M, Moreno M, Forns X, Bruguera M, Arroyo V, Brenner DA, Ginès P. Effects of losartan on hepatic expression of nonphagocytic NADPH oxidase and fibrogenic genes in patients with chronic hepatitis C. Am J Physiol Gastrointest Liver Physiol. 2009;297:G726-G734. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 171. | Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, Davion T, Oberti F, Broët P, Emilie D. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390-1397. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in RCA: 316] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 172. | Germano V, Picchianti Diamanti A, Baccano G, Natale E, Onetti Muda A, Priori R, Valesini G. Autoimmune hepatitis associated with infliximab in a patient with psoriatic arthritis. Ann Rheum Dis. 2005;64:1519-1520. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 173. | Marques M, Magro F, Cardoso H, Carneiro F, Portugal R, Lopes J, Costa Santos C. Infliximab-induced lupus-like syndrome associated with autoimmune hepatitis. Inflamm Bowel Dis. 2008;14:723-725. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 174. | Fairhurst DA, Sheehan-Dare R. Autoimmune hepatitis associated with infliximab in a patient with palmoplantar pustular psoriaisis. Clin Exp Dermatol. 2009;34:421-422. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 175. | Menon KV, Stadheim L, Kamath PS, Wiesner RH, Gores GJ, Peine CJ, Shah V. A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol. 2004;99:255-260. [PubMed] [Cited in This Article: ] |
| 176. | Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, Boardman L, Gores GJ, Harmsen WS, McClain CJ. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953-1960. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in RCA: 248] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 177. | Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637-1648. [PubMed] [Cited in This Article: ] |
| 178. | Gelfand EV, Cannon CP. Rimonabant: a selective blocker of the cannabinoid CB1 receptors for the management of obesity, smoking cessation and cardiometabolic risk factors. Expert Opin Investig Drugs. 2006;15:307-315. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 179. | McHutchison J, Goodman Z, Patel K, Makhlouf H, Rodriguez-Torres M, Shiffman M, Rockey D, Husa P, Chuang WL, Levine R. Farglitazar lacks antifibrotic activity in patients with chronic hepatitis C infection. Gastroenterology. 2010;138:1365-1373, 1373.e1-2. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 180. | Kang KW, Kim YG, Cho MK, Bae SK, Kim CW, Lee MG, Kim SG. Oltipraz regenerates cirrhotic liver through CCAAT/enhancer binding protein-mediated stellate cell inactivation. FASEB J. 2002;16:1988-1990. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 181. | Kim SG, Kim YM, Choi JY, Han JY, Jang JW, Cho SH, Um SH, Chon CY, Lee DH, Jang JJ. Oltipraz therapy in patients with liver fibrosis or cirrhosis: a randomized, double-blind, placebo-controlled phase II trial. J Pharm Pharmacol. 2011;63:627-635. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 182. | Weerachayaphorn J, Luo Y, Mennone A, Soroka CJ, Harry K, Boyer JL. Deleterious effect of oltipraz on extrahepatic cholestasis in bile duct-ligated mice. J Hepatol. 2014;60:160-166. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 183. | Vos TA, Van Goor H, Tuyt L, De Jager-Krikken A, Leuvenink R, Kuipers F, Jansen PL, Moshage H. Expression of inducible nitric oxide synthase in endotoxemic rat hepatocytes is dependent on the cellular glutathione status. Hepatology. 1999;29:421-426. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 184. | Oliveira CP, Alves VA, Lima VM, Stefano JT, Debbas V, Sá SV, Wakamatsu A, Corrêa-Giannella ML, de Mello ES, Havaki S. Modulation of hepatic microsomal triglyceride transfer protein (MTP) induced by S-nitroso-N-acetylcysteine in ob/ob mice. Biochem Pharmacol. 2007;74:290-297. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 185. | Ronis MJ, Butura A, Sampey BP, Shankar K, Prior RL, Korourian S, Albano E, Ingelman-Sundberg M, Petersen DR, Badger TM. Effects of N-acetylcysteine on ethanol-induced hepatotoxicity in rats fed via total enteral nutrition. Free Radic Biol Med. 2005;39:619-630. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 186. | Baumgardner JN, Shankar K, Hennings L, Albano E, Badger TM, Ronis MJ. N-acetylcysteine attenuates progression of liver pathology in a rat model of nonalcoholic steatohepatitis. J Nutr. 2008;138:1872-1879. [PubMed] [Cited in This Article: ] |
| 187. | Bémeur C, Vaquero J, Desjardins P, Butterworth RF. N-acetylcysteine attenuates cerebral complications of non-acetaminophen-induced acute liver failure in mice: antioxidant and anti-inflammatory mechanisms. Metab Brain Dis. 2010;25:241-249. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 188. | Setshedi M, Longato L, Petersen DR, Ronis M, Chen WC, Wands JR, de la Monte SM. Limited therapeutic effect of N-acetylcysteine on hepatic insulin resistance in an experimental model of alcohol-induced steatohepatitis. Alcohol Clin Exp Res. 2011;35:2139-2151. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 189. | Kawada N, Seki S, Inoue M, Kuroki T. Effect of antioxidants, resveratrol, quercetin, and N-acetylcysteine, on the functions of cultured rat hepatic stellate cells and Kupffer cells. Hepatology. 1998;27:1265-1274. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in RCA: 310] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 190. | Mato JM, Cámara J, Fernández de Paz J, Caballería L, Coll S, Caballero A, García-Buey L, Beltrán J, Benita V, Caballería J. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial. J Hepatol. 1999;30:1081-1089. [PubMed] [Cited in This Article: ] |
| 191. | de Oliveira CP, Stefano JT, de Siqueira ER, Silva LS, de Campos Mazo DF, Lima VM, Furuya CK, Mello ES, Souza FG, Rabello F. Combination of N-acetylcysteine and metformin improves histological steatosis and fibrosis in patients with non-alcoholic steatohepatitis. Hepatol Res. 2008;38:159-165. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 192. | Feld JJ, Modi AA, El-Diwany R, Rotman Y, Thomas E, Ahlenstiel G, Titerence R, Koh C, Cherepanov V, Heller T. S-adenosyl methionine improves early viral responses and interferon-stimulated gene induction in hepatitis C nonresponders. Gastroenterology. 2011;140:830-839. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 193. | Vercelino R, Crespo I, de Souza GF, Cuevas MJ, de Oliveira MG, Marroni NP, González-Gallego J, Tuñón MJ. S-nitroso-N-acetylcysteine attenuates liver fibrosis in cirrhotic rats. J Mol Med (Berl). 2010;88:401-411. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 194. | Czaja AJ. Promising pharmacological, molecular and cellular treatments of autoimmune hepatitis. Curr Pharm Des. 2011;17:3120-3140. [PubMed] [Cited in This Article: ] |
| 195. | Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6-20. [PubMed] [Cited in This Article: ] |
| 196. | Majano PL, Medina J, Zubía I, Sunyer L, Lara-Pezzi E, Maldonado-Rodríguez A, López-Cabrera M, Moreno-Otero R. N-Acetyl-cysteine modulates inducible nitric oxide synthase gene expression in human hepatocytes. J Hepatol. 2004;40:632-637. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 197. | Majano PL, García-Monzón C, García-Trevijano ER, Corrales FJ, Cámara J, Ortiz P, Mato JM, Avila MA, Moreno-Otero R. S-Adenosylmethionine modulates inducible nitric oxide synthase gene expression in rat liver and isolated hepatocytes. J Hepatol. 2001;35:692-699. [PubMed] [Cited in This Article: ] |
| 198. | Parola M, Leonarduzzi G, Biasi F, Albano E, Biocca ME, Poli G, Dianzani MU. Vitamin E dietary supplementation protects against carbon tetrachloride-induced chronic liver damage and cirrhosis. Hepatology. 1992;16:1014-1021. [PubMed] [Cited in This Article: ] |
| 199. | Parola M, Muraca R, Dianzani I, Barrera G, Leonarduzzi G, Bendinelli P, Piccoletti R, Poli G. Vitamin E dietary supplementation inhibits transforming growth factor beta 1 gene expression in the rat liver. FEBS Lett. 1992;308:267-270. [PubMed] [Cited in This Article: ] |
| 200. | Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15:1667-1672. [PubMed] [Cited in This Article: ] |
| 201. | Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485-2490. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 505] [Cited by in RCA: 466] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 202. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 2215] [Cited by in RCA: 2401] [Article Influence: 160.1] [Reference Citation Analysis (2)] |
| 203. | McCarty MF. Full-spectrum antioxidant therapy featuring astaxanthin coupled with lipoprivic strategies and salsalate for management of non-alcoholic fatty liver disease. Med Hypotheses. 2011;77:550-556. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 204. | Barak AJ, Beckenhauer HC, Junnila M, Tuma DJ. Dietary betaine promotes generation of hepatic S-adenosylmethionine and protects the liver from ethanol-induced fatty infiltration. Alcohol Clin Exp Res. 1993;17:552-555. [PubMed] [Cited in This Article: ] |
| 205. | Samara K, Liu C, Soldevila-Pico C, Nelson DR, Abdelmalek MF. Betaine resolves severe alcohol-induced hepatitis and steatosis following liver transplantation. Dig Dis Sci. 2006;51:1226-1229. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 206. | Abdelmalek MF, Angulo P, Jorgensen RA, Sylvestre PB, Lindor KD. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol. 2001;96:2711-2717. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in RCA: 283] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 207. | Abdelmalek MF, Sanderson SO, Angulo P, Soldevila-Pico C, Liu C, Peter J, Keach J, Cave M, Chen T, McClain CJ. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology. 2009;50:1818-1826. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 208. | Bataller R, Sancho-Bru P, Ginès P, Lora JM, Al-Garawi A, Solé M, Colmenero J, Nicolás JM, Jiménez W, Weich N. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology. 2003;125:117-125. [PubMed] [Cited in This Article: ] |
| 209. | Matsusaka T, Ichikawa I. Biological functions of angiotensin and its receptors. Annu Rev Physiol. 1997;59:395-412. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in RCA: 164] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 210. | Weber KT, Sun Y, Katwa LC, Cleutjens JP. Tissue repair and angiotensin II generated at sites of healing. Basic Res Cardiol. 1997;92:75-78. [PubMed] [Cited in This Article: ] |
| 211. | Weber KT, Sun Y, Katwa LC. Myofibroblasts and local angiotensin II in rat cardiac tissue repair. Int J Biochem Cell Biol. 1997;29:31-42. [PubMed] [Cited in This Article: ] |
| 212. | Katwa LC, Campbell SE, Tyagi SC, Lee SJ, Cicila GT, Weber KT. Cultured myofibroblasts generate angiotensin peptides de novo. J Mol Cell Cardiol. 1997;29:1375-1386. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 213. | Hirose A, Ono M, Saibara T, Nozaki Y, Masuda K, Yoshioka A, Takahashi M, Akisawa N, Iwasaki S, Oben JA. Angiotensin II type 1 receptor blocker inhibits fibrosis in rat nonalcoholic steatohepatitis. Hepatology. 2007;45:1375-1381. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in RCA: 174] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 214. | Jonsson JR, Clouston AD, Ando Y, Kelemen LI, Horn MJ, Adamson MD, Purdie DM, Powell EE. Angiotensin-converting enzyme inhibition attenuates the progression of rat hepatic fibrosis. Gastroenterology. 2001;121:148-155. [PubMed] [Cited in This Article: ] |
| 215. | Zimmermann H, Reichen J, Zimmermann A, Sägesser H, Thenisch B, Höflin F. Reversibility of secondary biliary fibrosis by biliodigestive anastomosis in the rat. Gastroenterology. 1992;103:579-589. [PubMed] [Cited in This Article: ] |
| 216. | Dunn MA, Cheever AW, Paglia LM, Kelly EP, Duvall RH, Andrade ZA, Goldner FH. Reversal of advanced liver fibrosis in rabbits with Schistosomiasis japonica. Am J Trop Med Hyg. 1994;50:499-505. [PubMed] [Cited in This Article: ] |
| 217. | Hammel P, Couvelard A, O’Toole D, Ratouis A, Sauvanet A, Fléjou JF, Degott C, Belghiti J, Bernades P, Valla D. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med. 2001;344:418-423. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in RCA: 281] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 218. | Powell LW, Kerr JF. Reversal of “cirrhosis” in idiopathic haemochromatosis following long-term intensive venesection therapy. Australas Ann Med. 1970;19:54-57. [PubMed] [Cited in This Article: ] |
| 219. | Falkmer S, Samuelson G, Sjölin S. Penicillamine-induced normalization of clinical signs, and liver morphology and histochemistry in a case of Wilson’s disease. Pediatrics. 1970;45:260-268. [PubMed] [Cited in This Article: ] |
| 220. | Soyer MT, Ceballos R, Aldrete JS. Reversibility of severe hepatic damage caused by jejunoileal bypass after re-establishment of normal intestinal continuity. Surgery. 1976;79:601-604. [PubMed] [Cited in This Article: ] |
| 221. | Muretto P, Angelucci E, Lucarelli G. Reversibility of cirrhosis in patients cured of thalassemia by bone marrow transplantation. Ann Intern Med. 2002;136:667-672. [PubMed] [Cited in This Article: ] |
| 222. | Kaplan MM, DeLellis RA, Wolfe HJ. Sustained biochemical and histologic remission of primary biliary cirrhosis in response to medical treatment. Ann Intern Med. 1997;126:682-688. [PubMed] [Cited in This Article: ] |
| 223. | Friedman SL, Bansal MB. Reversal of hepatic fibrosis -- fact or fantasy? Hepatology. 2006;43:S82-S88. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in RCA: 274] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 224. | Czaja AJ, Davis GL, Ludwig J, Taswell HF. Complete resolution of inflammatory activity following corticosteroid treatment of HBsAg-negative chronic active hepatitis. Hepatology. 1984;4:622-627. [PubMed] [Cited in This Article: ] |
| 225. | Kanzler S, Gerken G, Löhr H, Galle PR, Meyer zum Büschenfelde KH, Lohse AW. Duration of immunosuppressive therapy in autoimmune hepatitis. J Hepatol. 2001;34:354-355. [PubMed] [Cited in This Article: ] |
| 226. | Ferenci P, Fried MW, Shiffman ML, Smith CI, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J Hepatol. 2005;43:425-433. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in RCA: 383] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 227. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 2125] [Cited by in RCA: 2160] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 228. | Gleeson D, Heneghan MA. British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut. 2011;60:1611-1629. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 229. | Sonneveld MJ, Janssen HL. Pros and Cons of Peginterferon Versus Nucleos(t)ide Analogues for Treatment of Chronic Hepatitis B. Curr Hepat Rep. 2010;9:91-98. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 230. | Verma S, Gunuwan B, Mendler M, Govindrajan S, Redeker A. Factors predicting relapse and poor outcome in type I autoimmune hepatitis: role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol. 2004;99:1510-1516. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 231. | Miyake Y, Iwasaki Y, Terada R, Takagi S, Okamaoto R, Ikeda H, Sakai N, Makino Y, Kobashi H, Takaguchi K. Persistent normalization of serum alanine aminotransferase levels improves the prognosis of type 1 autoimmune hepatitis. J Hepatol. 2005;43:951-957. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 232. | Miyake Y, Iwasaki Y, Terada R, Okamaoto R, Ikeda H, Makino Y, Kobashi H, Takaguchi K, Sakaguchi K, Shiratori Y. Persistent elevation of serum alanine aminotransferase levels leads to poor survival and hepatocellular carcinoma development in type 1 autoimmune hepatitis. Aliment Pharmacol Ther. 2006;24:1197-1205. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 233. | Montano-Loza AJ, Carpenter HA, Czaja AJ. Improving the end point of corticosteroid therapy in type 1 autoimmune hepatitis to reduce the frequency of relapse. Am J Gastroenterol. 2007;102:1005-1012. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 234. | van Gerven NM, Verwer BJ, Witte BI, van Hoek B, Coenraad MJ, van Erpecum KJ, Beuers U, van Buuren HR, de Man RA, Drenth JP. Relapse is almost universal after withdrawal of immunosuppressive medication in patients with autoimmune hepatitis in remission. J Hepatol. 2013;58:141-147. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 235. | Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5:167sr1. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 455] [Cited by in RCA: 480] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 236. | Manns MP. Autoimmune hepatitis: the dilemma of rare diseases. Gastroenterology. 2011;140:1874-1876. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |