Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. Jan 7, 2014; 20(1): 148-162
Published online Jan 7, 2014. doi: 10.3748/wjg.v20.i1.148
Published online Jan 7, 2014. doi: 10.3748/wjg.v20.i1.148
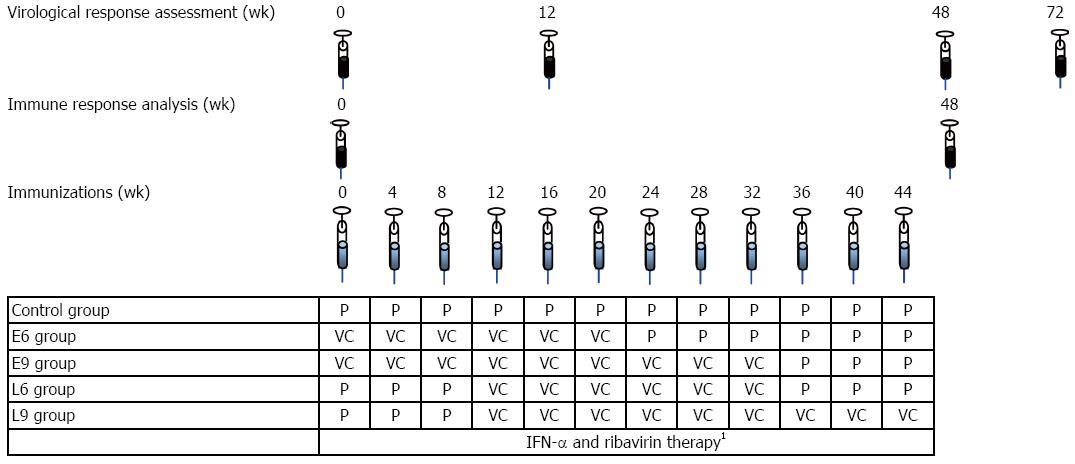
Figure 1 Study design.
148 wk of IFN-α-2b (3 × 106 units, subcutaneous, three times a week) plus ribavirin (1000 or 1200 mg daily, according to body weight)The control group (n = 30) received 12 vaccine placebo inoculations. Two groups received 6 inoculations of CIGB-230, one (n = 16) starting simultaneously with the antiviral treatment as early add-on (E6), and the other (n = 15) starting on week 12 of therapy as late add-on (L6). The remaining two groups were inoculated 9 times with CIGB-230, one (n = 16) as early add-on (E9) and the other (n = 15) as late add-on (L9). All inoculations relating the vaccine candidate or the placebo took place once every 4 wk. To maintain the blinding of the study, placebo administrations took place in the immunization groups, once every 4 wk, on weeks not corresponding to vaccine candidate administration, according to the immunization schedule. IFN: Interferon; VC: Vaccine candidate; P: Vaccine candidate’s placebo.
- Citation: Amador-Cañizares Y, Martínez-Donato G, Álvarez-Lajonchere L, Vasallo C, Dausá M, Aguilar-Noriega D, Valenzuela C, Raíces I, Dubuisson J, Wychowski C, Cinza-Estévez Z, Castellanos M, Núñez M, Armas A, González Y, Revé I, Guerra I, Pérez Aguiar &, Dueñas-Carrera S. HCV-specific immune responses induced by CIGB-230 in combination with IFN-α plus ribavirin. World J Gastroenterol 2014; 20(1): 148-162
- URL: https://www.wjgnet.com/1007-9327/full/v20/i1/148.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i1.148