INTRODUCTION
Wound injury and repair is one of the most common pathophysiological processes during human life. In all organ systems, the normal mammalian response to injury occurs in three overlapping but distinct stages: inflammation, new tissue formation, and tissue remodeling[1]. Therefore, injured organs, such as lung, kidney, liver or skin, will first respond with inflammation when insults, such as those caused by microorganisms and toxins, occur. Then next critical step is repair and regeneration, but during the repair or remodeling process, excessive deposition of extracellular matrix (ECM) collagen 1 and collagen 3 leads to hypertrophic scars, which results in tissue dysfunction[1]. Fibrosis is an excessive, uncontrolled injury response that occurs in organs, such as the lungs, kidneys, heart or skin, and is described by a persistent deposition of ECM. Fibrosis has been as a leading cause of morbidity and mortality[2]. There are more similarities between liver and skin regeneration due to the sustained regenerative capabilities of epidermal cells and hepatocytes after injury. Additionally, epidermal cells can be regenerated by remnant stem cells in hair follicles, and hepatocytes can be regenerated by cells in the canals of Hering, such as oval stem cells or other progenitors[1]. The core stage for injuries is the repair response and scar formation. As a protective response to insults, regeneration follows injury; therefore, the fibrogenetic source and the mechanism impeding regeneration are essential. In this review, we will explore recent evidence of liver fibrogenesis and possible regenerative capacities, especially in pathways that have been newly targeted to reverse fibrogenesis.
LIVER REGENERATION IN PHYSIOLOGICAL AND PATHOLOGICAL CONDITIONS
Unlike liver regeneration by Prometheus in Greek mythology, we have considered “regeneration” to be a hyperplasic response rather than the actual regeneration of the remnant liver[3-5]. After a 2/3 partial hepatectomy, most hepatic cells rapidly enter the cell cycle and undergo an average of approximately 1.6 cycles of replication per cell to completely restore the original liver mass[5,6]. The classic transplantation experiments using fumarylacetoacetate hydrolase (FAH)-deficient mice (FAH knockout mice) and urokinase-type plasminogen activator transgenic mice demonstrated that hepatocytes can replicate at least 69 times[7,8]. Interestingly, hepatocytes can differentiate into cholangiocytes and form mature bile ducts after bile duct ligation and toxic biliary injury and may even behave as stem cells under select circumstances[9,10]. However, under physiological conditions, hepatic regeneration only occurs to replace individual, aged hepatocytes-typically those in zone 1 (periportal)[11]. The regenerative components required for the process may be categorized into three networks: cytokine, growth factor and metabolic[12]. The innate immune system and cytokines, such as interleukin-6 (IL-6), tumor necrosis factor (TNF), hepatocyte growth factor (HGF) and complement, have been identified and recognized as playing important roles in regenerating the liver[12-14]. TNF binds its type I receptor, leading to nuclear factor kappa B activation in Kupffer cells, which produce IL-6 and TNF; IL-6 is subsequently released into the serum and binds to its receptor to activate the STAT-3 signaling pathway to initiate hepatocyte regeneration[12]. Transforming growth factor-beta (TGF-β) is released by the stellate cells, leading to Smad2 and Smad3 phosphorylation. Smad complexes translocate into the nucleus to transactivate target genes to induce epithelial to mesenchymal transition (EMT) and inhibit proliferation[15]. TGF-β may be an essential factor in liver fibrosis.
Activation of intrahepatic stem cells, such as hepatocyte progenitor cells and oval stem cells, and bone marrow stem cells are the main sources of exceptional regenerative capacity[16]. The properties of the liver generally allow for complete reconstitution following acute, moderate injuries.
After partial hepatectomy or CCl4-induced injury, liver regeneration is replicated by remanent hepatocytes. In rare cases, in the regeneration of the liver that injures induced by other toxins, such as galactosamine, activation of a progenitor cell compartment to replicate and differentiate[12].
However, the liver does not heal as effectively in response to chronic liver diseases such as liver fibrosis and cirrhosis.
LIVER FIBROSIS AND REGENERATION
Liver fibrosis is an excessive scar response leading to cirrhosis, which is characterized by the formation of regenerative nodules in the liver parenchyma separated by fibrotic septa. Three major mechanisms are involved in the generation of cirrhosis: cell death, aberrant extracellular matrix deposition (fibrosis), and vascular reorganization[17]. Liver fibrosis mainly represents quantitative and qualitative changes in the ECM, including the deposition of collagens, elastin, and tenacin, which can increase 3-5 fold[18]. The excessive deposition is accompanied by a shift in the type of ECM in the subendothelial space from the normal low-density, membrane-like, basement matrix to an interstitial-type matrix containing fibril-forming collagens[18]. Liver fibrosis accelerates to end-stage cirrhosis and shifts from reversible to irreversible. Therefore, most efforts should focus on treating liver fibrosis and understanding early fibrogenesis to control its progression. Activated fibroblasts or myofibroblasts are the key mediators of liver fibrosis[17,19,20]. Stellate cells, which are located in the subendothelium and constitute approximately 5% of the liver parenchyma, had been considered to represent the entire population of activated fibroblast cells; however, stellate cells have recently been found to possess more multifaceted functions in liver fibrosis, and we now know they are not the sole cause of fibrosis[19,21]. Such activated fibroblasts can be derived via the activation and proliferation of resident fibroblasts [hepatocyte stellate cells (HSCs) and portal fibroblasts], circulating fibrocytes, bone marrow stem or progenitor cells and epithelium-mesenchymal transition cells[2,19-22].
The underlying causes of diminished liver regeneration in liver fibrosis and cirrhosis are still a mystery. Using the proliferating cell nuclear antigen to assess proliferation, a cirrhotic liver has more DNA synthesis than a normal liver but also has great variability in the pseudolobules[23,24]. However, the high proliferation index does not reflect the actual cell division rates. The cell cycle is impaired by anaphase bridges, aberrant mitosis, and arrest in G2/M, which has been shown in mTR-/- transgenic mice[25]. Telomere shortening delays liver regeneration and can be restored by telomerase treatment, which has been shown to improve albumin levels in mTR-/- transgenic CCl4-cirrhosis models. Telomere shortening is thought to be proof that the replicative activity of hepatocytes is diminished in advanced cirrhosis and chronic liver injury in humans[26-28]. Telomere delivery inhibits the progression of cirrhosis in mice[25] and leads to a state of “replicative senescence”[29]. As with wound repair, the majority of hepatocytes are believed to undergo necrosis or apoptosis, providing space for proliferating cells during chronic insults, such as viral infections, that exhaust the regenerative capability of the liver. Telomere shortening is evidence of these processes. However, there is no direct evidence to support the loss of regenerative capability during chronic insults is entirely due to continuous regeneration and impairments. Therefore, we investigated possible regenerative mechanisms in fibrosis.
HEPATOCYTE EMT AND FIBROSIS
The most distinct difference in the injury response is the liver’s powerful regenerative capability compared to the kidneys, lungs and others organs. Another difference is the rapid progression in fibrosis due to acute processes, such as hepatitis C virus infection[30] or drug injury, or a chronic process, such as chronic hepatitis B virus (HBV) infection. Therefore, liver fibrosis is a more complex process relative to many other processes. The EMT is believed to play an important role in fibrosis, which may be reversed or attenuated by antagonizing essential cytokines and growth factors[31]. Undergoing an EMT refers to the loss of apicobasal polarity in epithelial cells; intercellular adhesion complexes undergo dramatic phenotypic changes, causing them to become nonpolar and thus allowing these cells to move through the ECM like mesenchymal cells[32-34]. Organ fibrosis can be classified as a type 2 EMT, which is associated with tissue repair and involves secondary epithelial or endothelial cells transitioning to resident tissue fibroblasts in response to persistent inflamemation[33,34]. A type 2 EMT can continue to respond to ongoing inflammation and lead to the expression of mesenchymal markers on cells, which can advance to various extents through an EMT, namely, a partial EMT. The partial EMT refers to an intermediate phenotype as cell transition, with progressive loss of epithelial markers (E-cadherin, ZO-1) and gain mesenchymal markers (vimentin, alpha smooth muscle actin, FSP1 and β-catenin)[33]. If the cells ultimately shed all of their epithelial markers and gain a complete fibroblastic phenotype, the cells have undergone a complete EMT[33]. The accumulating evidence has suggested that the EMT contributes to liver fibrosis, similar to processes that occur in other organs, such as the lungs, kidneys, and intestines[33].
An EMT can be found in response to growth factors, such as epidermal growth factor (EGF) or TGF-β, and dimethyl sulfoxide in rat fetal liver cells[35,36]. In normal mouse and adult livers, stroma cells can express both mesenchymal (vimentin, collagen I and alpha smooth muscle actin) and epithelial markers (cytokeratins, albumin and E-cadherin) during the hematopoietic but not the nonhematopoietic liver by the end of gestation, indicating that liver stroma cells may be EMT cells that support hematopoiesis[37]. Chronically damaged livers, as in cirrhosis, in which there are a large number of epithelial progenitors and myofibroblastic HSCs, have cells that undergo an EMT similar to the transition that occurs in the kidneys under pathological conditions[38,39]. Indirect evidence of EMT has been observed in HSC culture in vitro, in which HSCs were shown to coexpress mesenchymal and epithelial markers[38]. The primary HSC express stable mRNA levels of two epithelial markers, Mpk (an oval cell marker) and CK-19 (a marker of immature and mature biliary epithelial cells)[38]. Convincing evidence has shown that TGF-β can induce an EMT in mouse hepatocytes in vitro. The mechanism was demonstrated to be the result of TGF-β-induced activation of the Snail transcription factor, which is a key molecule in the EMT, and repression of epithelial markers, such as E-cadherin[32,40,41]. Further evidence has demonstrated, using AlbCre. R26RstoplacZ double transgenic mice in vivo, that hepatocytes can undergo EMT. Avoiding the double transgenic mice phenotype changes, using lineage-tracing experiments, hepatocytes can transdifferentiate into mesenchymal-like cells that have lost albumin and still have an activated Laz gene. After CCl4-induced liver fibrosis, up to 45% of FSP1-positive fibroblasts were found to be Laz(+), which drives the EMT, similar to processes that occur in the kidneys[39,42]. Undoubtedly, these transition cells have lost their hepatic markers, such as albumin, thus losing its main secreting function. Using collagen I and transferrin costaining demonstrated that half the resident hepatocytes had undergone an EMT phenotype in a TGF-β transgenic mouse model and in samples from patients with HBV, and the key transcription factor Snail was also found in the damaged regions[43]. Hepatitis C viral protein NS5A also induces hepatic dysplastic alterations of cell morphology with EMT phenotype and participates in oncogenic transformation of primary hepatocyte precursors[44]. TGF-β induces Snail, activates the Smad2/3 pathway and, finally, mediates the transition to the EMT phenotype[15]. The Snail-positive cells also consisted of 50% of the remaining hepatocytes in the damaged regions[43]. The mesenchymal markers vimentin and α-SMA were detected in fibrotic human and rat livers around the fibrotic septa, which indicates the presence of transition hepatocytes and EMT[45]. EMTs also occur in cirrhotic liver cells derived from murine CCl4-induced models[46]. Interestingly, the EMT-like cells express albumin and the mesenchymal marker vimentin and also gain collagen I secretary functions. Cell isolates from cirrhotic livers can exhibit anti-apoptosis effects in contrast to normal hepatocytes under TGF-β treatment[46]. While untreated normal liver-derived hepatocytes have been shown not to display features of an EMT, they did respond to TGF-β with increased vimentin expression and EMT characteristics[46]. Therefore, accumulated evidence has shown that EMT-like cells, even EMT cells, exist during chronic liver injury with a partial loss of functions such as albumin and transferrin secretion and a gain of mesenchymal features.
Recently, an EMT was also found in bile ducts in animal models and patients, especially in bile duct ligation, primary biliary cirrhosis and nonalcoholic fat liver diseases, due to hedgehog (Hh) signaling activation[47-49]. Hedgehog signaling is low in the normal adult liver but plays an important role in liver development[50]. Injured cholangiocytes activate hedgehog signaling through the patched receptor (Ptc) to release glioblastoma (Gli) family transcription factors to Hh-target genes. In Patched-deficient, Patched haplo-insufficient [Ptc(+/-)] and PtcLacZ mouse models, the bile ducts exhibit more EMTs, myofibroblast accumulation and fibrosis[48,49,51]. Hh activation in bile ducts can contribute to biliary fibrosis and cirrhosis. Different mesenchymal markers, such as vimentin, α-smooth muscle actin and S100A4, are located in the fibrous septa and extend around the nodules of liver parenchyma, which contain transition hepatocytes that have gained the ability to secrete ECM[43,46,48,49]. Based on the recent discoveries, these transition hepatocytes and cholangiocytes can be remodeled into other fibroblast-like phenotypes upon exposure to different insults and thus can be divided into two stages: partial-EMT or EMT-like and true, or complete, EMT. The partial-EMT can exist in the three stages of wound repair: inflammation, new tissue formation, and remodeling. Inflammation and necrosis can release different cytokines that initiate the clearance of the necrosis area and regeneration. The recognized cells contain EGF, IL-6, HGF, TNF and the important negative regulator TGF-β, especially in liver regeneration[1,12]. Until now, TGF-β, EGF and others through the TGF-β, Smad or hedgehog pathways initiate the EMT in different liver diseases and liver fibrosis in animal models[43,46,48-50]. Therefore, various cytokines and growth factors, released by necrotic cells, infiltrating inflammatory cells, HSCs and activated fibroblasts from different sources, can initiate the EMT as an early event during liver fibrosis.
However, these studies have been recently questioned because the use of transgenic-animal models allows for in vivo lineage-specific tracking using EMT-specific markers. These studies use a cross between an alpha-fetoprotein (AFP) cre mouse and a ROSA26YFP or ROSA26-β-gal stop mouse to trace cell fate through the expression of AFP[52,53]. None of the resulting fibrosis cells originated from the genetically marked hepatic and biliary epithelial cells. The challenging EMT results were repeated after inducing liver fibrosis by bile duct ligation (BDL), CCl4, or 3,5-diethoxycarbonyl-1,4-dihydrocollidine. However, the critical concerns originate from these EMT markers and the absence of such evidence in clinical patients.
FSP1 and Snail are popular markers for detecting the transition. FSP1 was not expressed by HSCs or type I collagen-producing fibroblasts in liver sections from FSP-GFP reporter mice BDL or CCl4-treated mice. FSP-1 is expressed in inflammatory macrophages, thus FSP1 is not a marker for myofibroblasts or their precursors[54]. Another important EMT marker, Snail, plays a key role in liver fibrosis progression in vivo in a CCl4 model by triggering the proximal genetic programs that control multiple aspects of fibrogenesis in the Snail transgenic CKO mouse, which promotes growth factor expression and extracellular matrix biosynthesis to the ensuing chronic inflammatory responses in liver fibrosis[55]. These Snail-positive cells also lose transferrin expression in the damaged regions, which is a specific liver marker in a complete epithelial to mesenchymal transition. The partially transdifferentiated hepatocytes comprised 50% of the remaining hepatocytes in the damaged regions, although they cannot be distinguished from other immune cells[43].
Although there is no current evidence that myofibro-blasts originate from liver cells, more experimental and clinical liver fibrosis were reported. These models may not reflect the pathophysiology in chronic human liver disease and in the TGF-β mouse model, which requires further study. Clearly, such studies have offered important evidence for the further study of EMT programs in liver fibrosis. Nevertheless, liver fibrosis may be independent of the hepatocyte EMT program and may instead be affected by important factors, such as Snail.
TARGETS OF LIVER FIBROSIS AND RECOVERING THE REGENERATIVE CAPACITY
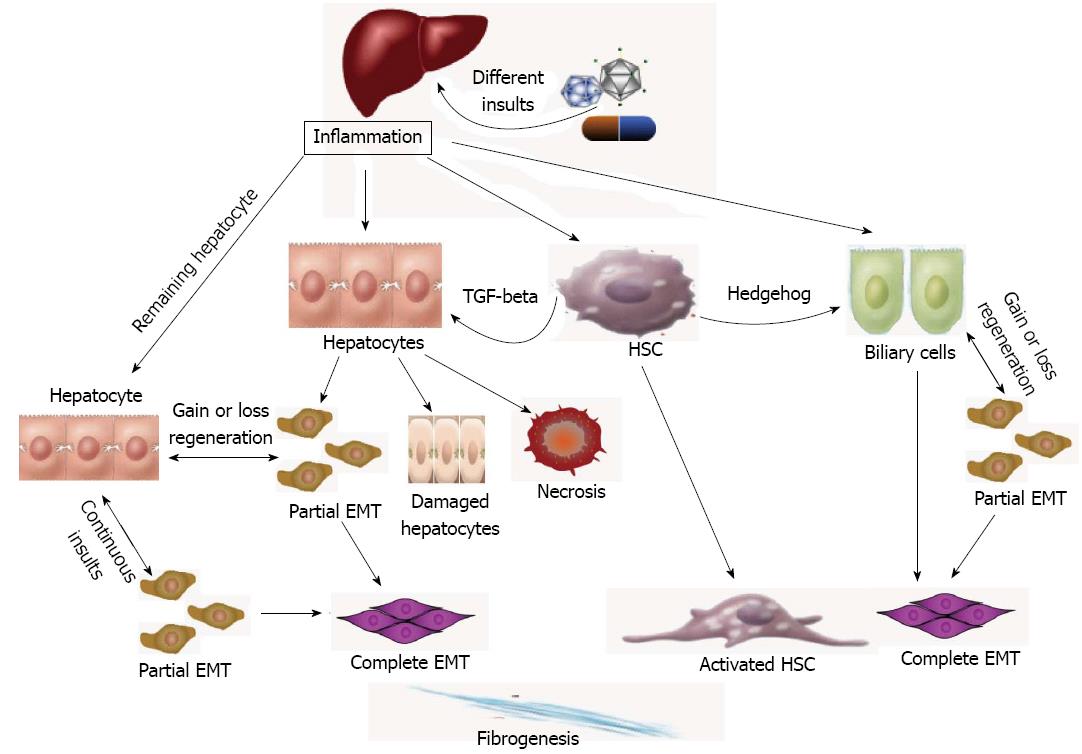
Stellate cells can be found within the progenitor cell niche in normal and regenerating livers, near the intrahepatic bile ducts, which was recognized as one of the central sources of fibrogenesis[21]. Recent studies have shown more important roles for immunity in promoting liver fibrogenesis[19]. Various cytokines and growth factors can affect stellate cells from inflammatory infiltrates and necrotic tissue. Stellate cells can also produce different cytokines or growth factors by paracrine or autocrine transmission to influence the adjacent hepatocytes. TGF-β, a key regulator of fibrogenesis and EMT, can be observed in acute or chronic liver injury in HSCs[13,56,57]. TGF-β can directly activate adjacent hepatocytes through Smad signaling, induce EMTs, and induce ECM and fibrogenesis. Hedgehog signaling may also be a key regulator in the promotion of EMT and fibrogenesis in bile duct diseases. Blocking these signaling pathways is an important therapeutic target for mediating regeneration (Figure 1).
Figure 1 Liver regeneration and the epithelial to mesenchymal transition.
The diagram shows the fibrogenesis via epithelial to mesenchymal transition (EMT) in the liver. Different insults initiate inflammation and then cause hepatocyte stellate cells (HSCs) activation and hepatocyte and biliary cell damage, necrosis and EMT. Except for the necrotic and damaged cells, the remaining “normal” liver cells can be divided into 2 groups: normal and EMT-like hepatocytes. Continuous insults will shift those EMT-like cells to complete EMT cells and finally myofibroblasts, the main producer of extracellular matrix, which may be one of the main causes of an early loss of regenerative capacity. A similar process also occurs in biliary cells. HSCs play an important role in secreting key cytokines, such as transforming growth factor-beta (TGF-beta) and hedgehog, to affect the adjacent cells and promote EMTs.
TGF-β exerts its effects by binding to the TGF-β type II receptor, which causes recruitment and phosphorylation of receptor type I and formation of a complex. The activated receptor type I subsequently recruiting Smad2/3 and Smad4, which are known intracellular mediators of TGF-β. Once phosphorylated by the activated TGF-β receptor, Smad2 and/or Smad3 bind Smad4 and translocate to the nucleus, where they regulate TGF-β target genes[57]. Smad7, a feedback regulator of TGF-β, can prevent liver fibrosis and HSC activation in rat BDL- and CCL4-induced liver fibrosis models[15,58]. Using Smad transgenic mice also attenuates TGF-β signaling and EMTs, thus improving CCl4-provoked liver damage and fibrosis[43]. The complex formation of BMP-7 and TGF-β with different types of ALK receptors is mediated by Smad proteins. In renal tubular epithelial cells and mammary ductal epithelial cells, BMP7 has been shown to reverse the TGF-β1-induced EMT, given that NTN mice (nephrotoxic serum nephritis; a chronic nephritis model) treated with recombinant human BMP can re-induce E-cadherin[59].
The Hh signaling that was observed in bile duct fibrosis and EMTs has provided us with a new mechanism and new therapeutic targets[47,51]. Hh ligands that interact with the Hh receptor Patched (Ptc) liberate the coreceptor Smoothened, which activates Gli family transcription factors and Hh-target genes[50]. The Hh pathway can be activated in the liver after BDL and Roux-en-Y hepaticojejunostomy to relieve biliary obstructions. Hh signaling decreased as the duct populations and concomitant fibrosis were observed[48,60]. The role of the Hh pathway was observed in nonalcoholic fatty liver disease and verified in Ptc transgenic mouse bile duct cells. The Hh inhibitor cyclopamine and the Hh-neutralizing antibody have been shown to reverse the EMT and fibrosis in mouse models and in vitro co-culture with cholangiocytes and MF-HSCs and also to reduce TGF-β expression[48,49].
Similar to TGF-β, activated HSCs can produce an Hh ligand by paracrine and autocrine signaling[48,61,62]. Therefore, HSC can produce TGF-β and the Hh ligand to affect the adjacent hepatocytes and cholangiocytes to induce EMT and fibrosis. Inhibiting the Hh pathway may reduce TGF-β expression, which may indicate a communication between them[49].
If the remaining “normal” liver cells had 40%-50% EMTs, as mentioned above, and continuously were damaged, the hepatocytes capable of regeneration may actually be less than 50%, excluding the damaged or necrotic hepatocytes, which would lose their normal functions and exhaust their regenerative capacity earlier than expected. The remaining hepatocytes, including the newly identified partial EMT or EMT-like cells, have diminished regenerative capacity potentially due to elevated rates of apoptosis followed by repeated regeneration and damage according to the traditional theory[13]. The newly regenerated hepatocytes and the remaining hepatocytes can continuously transition to mesenchymal cells during fibrosis (Figure 1). Recognizing whether these phenotype changes or the transition cells have a more important role in targeting therapy for controlling the transition phenotype can not only disrupt the transition to produce ECM but can also help to recover the regenerative capacity of the EMT-like cells.
As mentioned above, EMT-targeted therapy can reverse partial EMT cells, not only during fibrosis but also during regeneration. These phenotype changes or transition cells may provide us a new insight in identifying the regenerative capacity of these partial EMT or complete EMT cells and also for targeting new therapies.