Published online Feb 21, 2013. doi: 10.3748/wjg.v19.i7.1104
Revised: January 8, 2013
Accepted: January 17, 2013
Published online: February 21, 2013
Processing time: 108 Days and 11 Hours
AIM: To evaluate the safety and efficacy of granulocyte-colony stimulating factor (G-CSF) therapy in patients with hepatitis B virus (HBV)-associated acute-on-chronic liver failure (ACLF).
METHODS: Fifty-five patients with HBV-associated ACLF were randomized into two groups: the treatment group and the control group. Twenty-seven patients in the treatment group received G-CSF (5 μg/kg per day, six doses) treatment plus standard therapy, and 28 patients in the control group received standard therapy only. The peripheral CD34+ cell count was measured consecutively by flow cytometry. Circulating white blood cell count, biochemical parameters, and other clinical data of these patients were recorded and analyzed. All patients were followed up for a period of 3 mo to evaluate the changes in liver function and survival rate.
RESULTS: The peripheral neutrophil and CD34+ cell counts in the G-CSF group increased on day 3 from the onset of therapy, continued to rise on day 7, and remained elevated on day 15 compared to those of the control group. Child-Turcotte-Pugh score of patients in the treatment group was improved on day 30 from the onset of G-CSF therapy, compared to that in the controls (P = 0.041). Model for End-Stage of Liver Disease score of patients in the treatment group was improved on day 7 (P = 0.004) and remained high on day 30 from the onset of G-CSF therapy (P < 0.001) compared to that in controls. After 3 mo of follow-up observation, the survival rate in the treatment group (48.1%) was significantly higher than that in the control group (21.4%) (P = 0.0181).
CONCLUSION: G-CSF therapy promoted CD34+ cell mobilization in patients with HBV-associated ACLF, and improved the liver function and the survival rate of these patients.
- Citation: Duan XZ, Liu FF, Tong JJ, Yang HZ, Chen J, Liu XY, Mao YL, Xin SJ, Hu JH. Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World J Gastroenterol 2013; 19(7): 1104-1110
- URL: https://www.wjgnet.com/1007-9327/full/v19/i7/1104.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i7.1104
Hepatitis B virus (HBV) infection is one of the major public health problems. It is estimated that over 350 million individuals are chronically infected with HBV worldwide[1]. Some of these patients develop severe liver diseases such as acute-on-chronic liver failure (ACLF), liver cirrhosis, and hepato-cellular carcinoma[2]. The mortality rate of HBV-associated ACLF varies between 70%-80%[3,4]. Liver transplantation is the only effective treatment currently available, but its application is limited by the shortage of donors and the high cost.
In order to overcome these problems, alternative approaches have been proposed, such as an artificial liver support system[5], liver cell transplantation[6] and stem cell transplantations[7]. In particular, the great potential of stem cells to differentiate into multiple cell lineages raises the exciting hypothesis that these cells can be used in tissue repair and tissue-specific cell regeneration, when tissue-resided stem cells are not sufficient for the regeneration of a failing organ.
During liver regeneration, bone marrow-derived hematopoietic stem cells (HSC) may mobilize to the liver and, together with hepatocytes and intrahepatic stem cells, contribute to the proliferation of liver cells[8]. Hepatocytes carrying a Y chromosome were detected in the livers of female recipients who had received bone marrow transplantation from male donors[9]. Granulocyte-colony stimulating factor (G-CSF) can be used to mobilize stem cells to the periphery in patients with advanced liver disease, or to promote adequate numbers of cells for further transplantation. The safety and efficacy of this protocol has been investigated[10]. In experimental animal models, G-CSF ameliorated liver injury and improved the survival rate in rats with D-galactosamine-induced acute liver failure[11,12]. When administered to patients with severe liver cirrhosis, G-CSF boosted the numbers of peripheral leukocytes and CD34+ bone marrow-derived HSCs[13]. Therefore, G-CSF therapy may be beneficial for liver regeneration in patients with different kinds of liver injuries.
Our objective in this clinical study was to evaluate the effects of G-CSF therapy on the proliferation of peripheral CD34+ cells and on liver function in patients with HBV-associated ACLF. The parameters of liver function in these patients were consecutively measured. Child-Turcotte-Pugh (CTP) score, Model for End Stage of Liver Disease (MELD) score and survival rate of these patients were evaluated during a 3-mo follow-up study.
A total of 55 patients with HBV-associated ACLF were recruited to our center from June 2009 to May 2011. HBV-associated ACLF, defined by the Asian Pacific Association for the Study of the Liver Working Party[2], is an acute hepatic insult manifested as jaundice (serum bilirubin ≥ 5 mg/dL) and coagulopathy [international normalized ratio (INR) ≥ 1.5 or prothrombin activity < 40%], with complications of ascites and/or encephalopathy within 4 wk in patients previously diagnosed or undiagnosed with chronic liver disease. Other inclusion criteria included: (1) the presence of hepatitis B surface antigen in the serum for at least 6 mo; (2) the evidence of active viral replication as indicated by detectable HBV DNA in the serum (≥ 1 × 104 copies/mL); (3) flares of hepatitis, marked by increased serum alanine aminotransferase (ALT) level to more than 5-fold of the upper limit of normal value; and (4) age between 18 to 65 years.
The exclusion criteria included the following: (1) super-infection or co-infection with hepatitis A, C, D, E, Epstein-Barr virus, cytomegalovirus, or human immunodeficiency virus; (2) a previous course of any antiviral, immuno-modulator or cytotoxic/immunosuppressive therapy for chronic hepatitis within the prior 12 mo; (3) evidence of decompensated liver disease prior to the enrollment; (4) hepato-cellular carcinoma diagnosed by computed tomography or magnetic resonance imaging; (5) co-existence of any other serious medical illnesses or other liver diseases such as autoimmune hepatitis, alcoholic liver disease, drug-induced liver injury or Wilson’s disease; (6) any concurrent evidence of sepsis; (7) malignant jaundice induced by obstructive jaundice and hemolytic jaundice; and (8) prolonged prothrombin time due to blood system disease.
This was a randomized, controlled, and double-blinded study. The sample size was determined as follows: Based on the hypothesis that G-CSF therapy can improve survival rate by 10% in the treatment group compared to the control group, with a power of 95% and an alpha error of 5%, the number of patients should be 25 in each group. A randomization number code was generated for each patient. Based on this code, each patient was assigned to receive G-CSF therapy plus standard medical therapy (G-CSF group), or standard medical therapy alone (control group). Both the patients and the investigators were blinded to the treatment regimen. The patients in the G-CSF treatment group received G-CSF (SL Pharm, Beijing, China) subcutaneously at the dosage of 5 μg/kg per day for six consecutive days, and were monitored with daily physical examination and laboratory tests. All patients received entecavir (0.5 mg/d, Squibb Pharmaceuticals Ltd., Shang Hai, China) and standard therapy (including reduced glutathione, glycyrrhizin, ademetionine, polyene phosphatidylcholine, alprostadil, and human serum albumin) on the day of admission. The white blood cell (WBC) counts were assessed twice per week in the first two weeks. In addition, abdominal ultrasound examination was performed on days 1 and 7 to evaluate the diameters of spleen and the hepatic portal vein.
CD34+ cells in the peripheral venous blood were measured consecutively twice per week in the first two weeks in all patients. Briefly, small aliquots of peripheral blood were incubated with PE-conjugated anti-CD34 monoclonal-antibody (BD Company, United States) for 30 min on ice. Erythrocytes were lysed with ammonium chloride for 10 min at room temperature. Cells were washed with phosphate-buffered saline, and kept on ice till flow cytometric analysis.
All patients had daily follow-ups and physical examinations in the first month, and then at least weekly for the next 2 mo. During the 3-mo follow-up period, patients were monitored for the following parameters: the levels of serum bilirubin and albumin, prothrombin time and concentration, INR, the levels of ALT and aspartate aminotransferase, the levels of blood urea and serum creatinine, complete blood analysis, estimation of the degree of ascites, CTP score, MELD score, and hepatic encephalopathy. The survival rates were evaluated over a period of 3-mo.
Sera from patients were collected and direct polymerase chain reaction (PCR) sequencing was used to screen for HBV reverse transcriptase (RT) domain if serum HBV DNA tests were positive after patients received entecavir therapy. The HBV gene fragment (nt 54-1278) encompassing the complete RT gene (nt 130-1161) was amplified by nested PCR. The primers and reaction conditions have been described previously[14]. Substitutions at positions rt180, rt184, rt202, rt204 and rt250 were taken as resistance mutations for analysis.
The protocol of our study is in compliance with the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the Clinical Research Ethics Committee of the Beijing 302 Hospital. All patients or their relatives in the G-CSF treatment group provided written informed consent prior to the enrollment.
Data were compiled using Excel XP and processed using Statistical Package for Science and Society (SPSS) version 12.0 (SPSS Inc., Chicago, IL). All quantitative variables were presented as mean ± SD. All qualitative data were described as frequency or percentage. Comparisons between groups for qualitative data were carried out using χ2 test, Fischer’s exact test, or McNemar test when appropriate. Independent sample t test and paired sample t test were used for quantitative variables with normal distribution, whereas non-parametric Mann-Whitney test and Wilcoxon signed-rank test were used for quantitative variables with non-normal distribution. In all tests, P < 0.05 were considered as statistically significant.
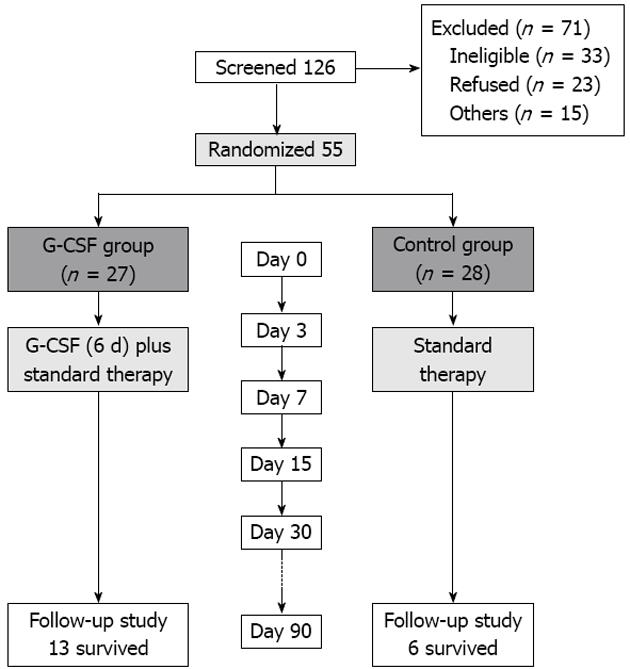
Of the 126 patients screened, 71 were excluded and 55 patients enrolled in our study. Among them, 27 were randomized to receive G-CSF therapy, and the other 28 were included as controls (Figure 1).
The two groups showed no statistical differences in gender, age, the baseline values of peripheral WBC, platelets, and other parameters (Table 1). All patients had a history of chronic hepatitis B, and had not received anti-viral therapy prior to the enrollment.
| Parameters | G-CSF group | Control group | P value |
| Gender (male %) | 22 (81.5) | 22 (78.6) | 0.755 |
| Age (yr) | 43.5 (29-63) | 45.9 (22-65) | 0.332 |
| WBC (109/L) | 5.79 ± 1.81 | 6.61 ± 1.71 | 0.443 |
| Neutrophil (109/L) | 3.53 ± 1.46 | 3.82 ± 1.17 | 0.114 |
| Platelets (109/L) | 182 (147-215) | 174 (149-175) | 0.680 |
| ALT (U/L) | 276 (197-801) | 252 (189-1239) | 0.430 |
| AST (U/L) | 246 (195-788) | 251 (187-980) | 0.544 |
| Total bilirubin (μmol/L) | 336 (181-519) | 320.0 (174.5-519.8) | 0.605 |
| Cr (μmol/L) | 83.8 ± 16.9 | 85.4 ± 53.87 | 0.475 |
| INR | 2.11 ± 0.28 | 2.34 ± 0.34 | 0.606 |
| ALB (g/L) | 29.11 ± 4.05 | 28.75 ± 4.63 | 0.596 |
| HBV DNA (log10) | 5.11 ± 1.37 | 5.55 ± 1.59 | 0.280 |
| CTP score | 12.17 ± 1.47 | 12.25 ± 1.29 | 0.349 |
| MELD score | 25.11 ± 3.30 | 26.30 ± 4.12 | 0.588 |
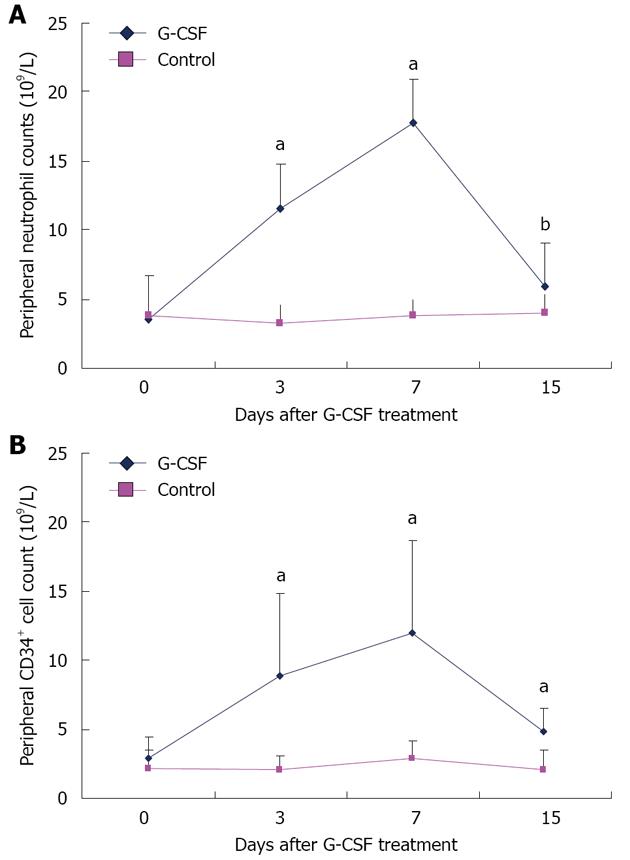
The baseline value of the circulating neutrophil count was compared between the G-CSF and the control groups (Table 1). No statistical difference was found between the baselines of the two groups (P = 0.443). However, on day 3 from the onset of G-CSF therapy, the peripheral neutrophil count increased to (11.59 ± 6.40) × 109/L, significantly higher than that of the control group (3.29 ± 1.25) × 109/L (P < 0.001). It continued to rise on day 7 to (17.76 ± 10.07) × 109/L in the G-CSF group, significantly higher than (3.82 ± 1.17) × 109/L in the control group (P < 0.001). On day 15, the neutrophil count decreased to (5.88 ± 3.69) × 109/L in the G-CSF group, but was still higher than (4.02 ± 1.33) × 109/L in the control group (P = 0.032) (Figure 2A).
Prior to G-CSF therapy, circulating CD34+ cell counts were comparable in the two groups - (2.97 ± 1.52) × 106/L in the G-CSF group and (2.23 ± 1.29) × 106/L in the control group (P = 0.085). On day 3 of treatment, circulating CD34+ cell counts in the G-CSF group increased to (8.96 ± 5.97) × 106/L, compared to (2.09 ± 1.02) × 106/L in the control group (P < 0.001). On day 7, the circulating CD34+ cell counts continued to rise to (12.05 ± 6.70) × 106/L, compared to (2.97 ± 1.22) × 106/L in the control (P < 0.001). On day 15, the CD34+ cell counts decreased to (4.92 ± 1.63) × 106/L in the G-CSF treatment group, still significantly higher than that in the control group (2.11 ± 1.39) (P < 0.001) (Figure 2B). On day 30, the CD34+ cell counts were nearly at the same level between the two groups.
In the course of the G-CSF therapy, all patients demonstrated good tolerance. Some patients were affected by minor side effects, such as fever (8 cases), headache (5 cases), and nausea (4 cases), but all the side effects resolved within 5 d after the withdrawal of G-CSF treatment.
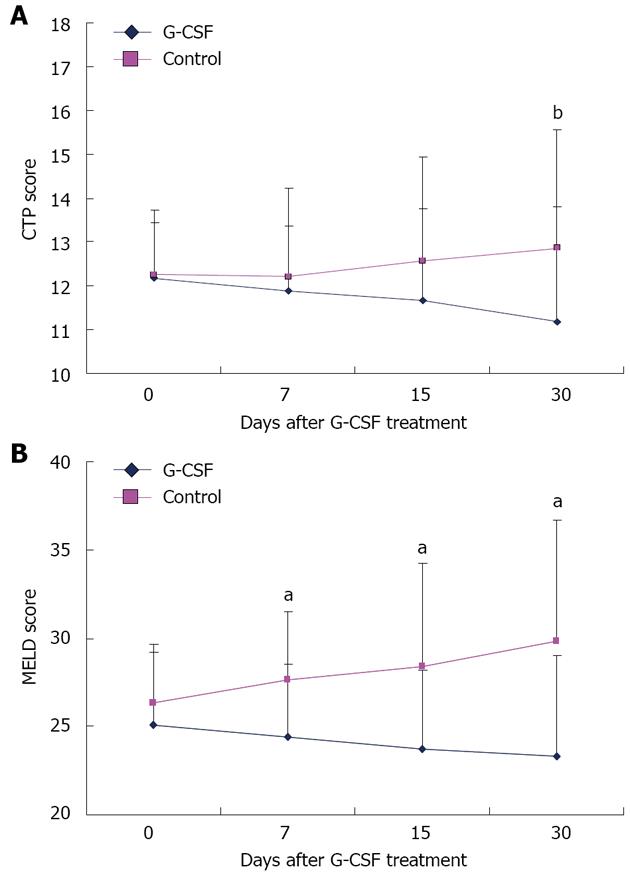
Prior to the G-CSF therapy, the CTP score was 12.17 ± 1.47 in the G-CSF group, and 12.25 ± 1.29 in the control group (P = 0.841). The CTP score decreased gradually after the G-CSF therapy, and reached 11.17 ± 2.76 on day 30 of treatment in the G-CSF group, compared to 12.86 ± 2.63 in the control group (P = 0.041) (Figure 3A).
The MELD score demonstrated an early decrease in the G-CSF group (Figure 3B). On day 7 of the therapy, the MELD score decreased to 24.4 ± 3.9 in the G-CSF group, compared to 27.6 ± 4.1 in the control group (P = 0.004). On day 15, the MELD score was 23.7 ± 5.8 in the G-CSF group, compared to 28.4 ± 4.5 in the control group (P < 0.001). This trend continued during the 30-d observation period (23.3 ± 6.9 vs 29.8 ± 5.7) (P < 0.001).
All patients enrolled in our study received entecavir antiviral treatment throughout the course of the study. After 3 mo of treatment, all the surviving patients demonstrated effective antiviral results. Of the 19 surviving patients, 16 (11 from G-CSF group, and 5 from control group) were detected negative for serum HBV DNA (below 102 copies/mL), and 3 (2 from G-CSF group and 1 from control group) were positive, but the concentrations of HBV DNA were all below 103 copies/mL. Serum HBV mutations were determined by direct sequencing methods and an entecavir-resistant mutation was not detected in these patients. No serious adverse effects of entecavir were observed in all patients.
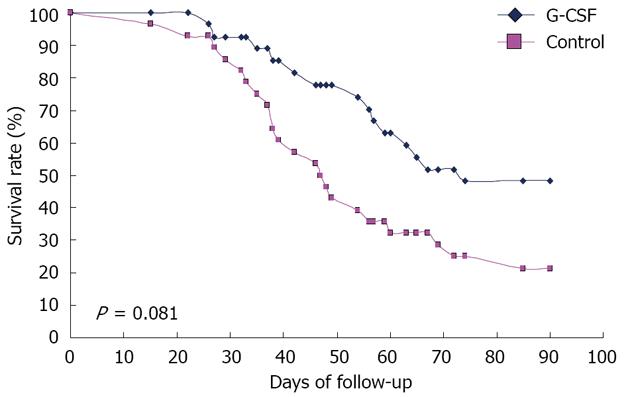
The patients’ survival rates were evaluated at the end of 3 mo (Figure 4). In the G-CSF group, 13 of 27 patients survived, but only 6 of 28 patients survived in the control group (χ2 value 5.584, P = 0.0181). All the patients had complications of ascites and electrolyte disturbances. In the treatment group, 4 patients died of encephalopathy, 5 of gastric intestinal bleeding, 2 of hepato-renal syndrome (HRS), and 3 of sepsis. In the control group, 6 died of encephalopathy, 3 of gastric intestinal bleeding, 6 died from HRS, and 7 of sepsis. More patients in the control group died of sepsis and HRS compared to those in the G-CSF group (χ2 value 4.863, P = 0.027).
In China, HBV-associated ACLF accounts for 85%-95% of all liver failure. Approximately 60% of the patients die from complications due to the lack of early liver transplantation[15,16]. Increasing evidence indicates that stem cells contribute to hepatic regeneration, which is essential for the restoration of liver function and thus the survival of the patients. Stem cells have been shown to induce the repairing process after acute liver injuries[10,12]. Therefore, G-CSF therapy may be a promising approach in the clinical treatment of patients with ACLF.
Some previous findings on the efficacy of G-CSF in patients with liver failure remain controversial. Di Campli et al[17] investigated the safety and efficacy of G-CSF in patients with ACLF. They found an increased number of CD34+ cells in the peripheral blood, down-regulated expression of C-X-C chemokine receptor type 4, very late activation of antigen 4 and vascular endothelial growth factor receptor in the G-CSF treatment group, but they did not show the effect of G-CSF on the survival rates of these patients. Similarly, Spahr et al[18] investigated the efficacy of G-CSF in patients with alcoholic steatohepatitis, and observed an elevated peripheral CD34+ cell count and proliferating hemopoietic cells in the liver tissues, although they failed to demonstrate the improvement of liver function. Recently Garg et al[19] also investigated the efficacy of G-CSF in ACLF patients with promising results. Sixteen of the 23 patients in the G-CSF group survived for 60 d, compared to merely 7 of the 24 in the control group. The discrepancies between the results by different groups may be attributable to the background of the enrolled patients. For example, Di Campli enrolled patients with alcohol liver failure, whereas Garg recruited patients with various types of liver diseases, including alcoholic-related liver diseases and HBV-associated ACLF.
In our current study we selected patients with HBV-associated ACLF for investigation. This is the first report to apply G-CSF therapy to patients with chronic HBV infection. All the patients were positive for serum HBV DNA and received entecavir antiviral treatment after enrollment. The two groups showed no statistically significant differences in gender, WBC count, CTP score and MELD score prior to the onset of the G-CSF therapy.
We observed that G-CSF therapy increased the peripheral neutrophil count and CD34+ cell count in patients with HBV-associated ACLF. In addition, the G-CSF treatment group demonstrated improved liver function compared to the control group, as demonstrated by the CTP and MELD scores. Our finding on survival rate was in consistency with that of Garg et al[19].
It is plausible to speculate that CD34+ HSC migrated from the bone marrow to the liver, and contributed to the regeneration of the liver. Future studies, such as liver biopsy or autopsy, may provide evidence for the mobilization of the stem cells. In these patients, MELD score improved sooner than CTP score after G-CSF therapy, but the mechanism is yet to be elucidated.
Currently, there are two classical pathways for stem cells to travel from the bone marrow to the liver. The first is G-CSF mobilization. Secondly, CD34+ cells can be isolated from bone marrow, purified with magnetic columns, and then re-injected into the liver through the hepatic artery or the portal vein[13,20]. However, patients with ACLF often have coagulation disorders; therefore, it is risky to apply the aforementioned complicated procedures. It is a reasonable argument that the approach of G-CSF administration is probably easier and less risky to implement than the separation, purification, and autologous transplantation of bone marrow-derived HSC cells to ACLF patients. Furthermore, it has been reported that contamination by red blood cells during CD34+ cell isolation can impair the efficacy of autologous HSC therapy[21].
Antiviral therapy has been deemed as an important therapy for patients with HBV-associated ACLF[2,22]. Previously, Huang et al[16] reported that antiviral therapy decreased the mortality rate in patients with HBV-associated ACLF, but the patients recruited to their study had lower ALT levels and higher prothrombin activity (over 30%), which may explain the different results between these two studies. In our study, the mortality rate in the control group was higher (78.6%) than those reported in the literature. This may be attributable to several factors. Firstly, the patients in our study had larger MELD scores, of 25 to 29, indicating more serious conditions. Secondly, most patients enrolled in our study had middle-stage (PA between 20%-30%) or late-stage ACLF (PA below 20%) according to the standards of the Chinese classification system[23], which may contribute to an increased mortality rate. Thirdly, antiviral therapy can suppress HBV replication; however, there may not be enough time for HBV suppression and liver function recovery to occur due to the short life expectancy in ACLF patients. A similar phenomenon was also observed by other researchers[24]. We also consider the possibility of HBV viral breakthrough in these patients. Serum HBV DNA mutations were determined by a direct sequencing method when serum HBV DNA was positive in patients, but we did not find any entecavir-resistant mutations. Although we did not find poor compliance in these patients, we could not exclude the possibility. In our study, all the patients received antiviral therapy to help mitigate additional hepatic insult and even liver failure caused by the re-activation of HBV.
Patients treated with G-CSF not only demonstrated a significantly better 90 d survival rate, CTP and MELD scores, but were also less likely to develop HRS and sepsis compared to the controls. This could be explained by increased numbers of neutrophils in these patients. Neutrophil dysfunction has been shown to cause sepsis and further the development of HRS in patients with ACLF[25]. We did not find a correlation between CD34+ cell number and liver function improvement, which means cell numbers, micro-environment of liver tissue and other possible factors may work together to improve liver function. That mechanism needs to be further investigated.
In conclusion, this randomized, controlled study clearly demonstrated the clinical safety and efficacy of G-CSF therapy in patients with HBV-associated ACLF. The convenience of administration makes G-CSF therapy readily implementable in large-scale, multi-center clinical sites, and further supports its benefits in restoring hepatic function and improving survival rate.
Hepatitis B virus-associated acute-on-chronic liver failure (HBV-ACLF) is associated with a high mortality. Liver transplantation could significantly improve the survival rate, but is limited by many factors, especially donor shortages. To overcome these problems, alternative approaches have been proposed. Granulocyte-colony stimulating factor (G-CSF) can be used to mobilize stem cells from bone marrow to the periphery, and then to the liver, in patients with advanced liver disease, and promote liver regeneration.
In experimental animal models, G-CSF ameliorated liver injury and improved survival rate in rats with D-galactosamine-induced acute liver failure. When administered to patients with severe liver cirrhosis, G-CSF boosted the numbers of peripheral leukocytes and CD34+ bone marrow-derived hematopoietic stem cells. Therefore, G-CSF therapy may be beneficial for liver regeneration in patients with different kinds of liver injuries. The safety and efficacy of a G-CSF protocol has been investigated but the efficacy is still controversial in patients with liver diseases.
In the current study, the authors selected patients with HBV-associated ACLF for investigation. This is the first randomized trial to apply G-CSF therapy to patients with chronic HBV infection. The authors observed that G-CSF therapy increased peripheral neutrophil count and CD34+ cell count in patients with HBV-associated ACLF. In addition, the G-CSF treatment group demonstrated improved liver function and survival rate compared to the control group.
HBV-associated ACLF patients treated with G-CSF not only demonstrated a significantly better 90 d survival rate, and improved CTP and MELD scores, but were also less likely to develop HRS and sepsis compared to the controls. The convenience of administration makes G-CSF therapy readily implementable in large-scale, multi-center clinical sites to further support its benefits in restoring hepatic function and improving survival rate.
HBV-associated ACLF, defined by the Asian Pacific Association for the Study of the Liver Working Party, is an acute hepatic insult manifested as jaundice (serum bilirubin ≥ 5 mg/dL) and coagulopathy (international normalized ratio ≥ 1.5 or prothrombin activity < 40%), with complications of ascites and/or encephalopathy within 4 wk in patients previously diagnosed or undiagnosed with chronic liver disease.
This is an interesting study in patients with decompensated hepatitis B patients.
P- Reviewer Spahr L S- Editor Song XX L- Editor Logan S E- Editor Xiong L
| 1. | Tujios SR, Lee WM. New advances in chronic hepatitis B. Curr Opin Gastroenterol. 2012;28:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, de Silva HJ, Hamid SS, Jalan R, Komolmit P. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL). Hepatol Int. 2009;3:269-282. [RCA] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 642] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 3. | Chan HL, Tsang SW, Hui Y, Leung NW, Chan FK, Sung JJ. The role of lamivudine and predictors of mortality in severe flare-up of chronic hepatitis B with jaundice. J Viral Hepat. 2002;9:424-428. [PubMed] |
| 4. | Tsubota A, Arase Y, Suzuki Y, Suzuki F, Sezaki H, Hosaka T, Akuta N, Someya T, Kobayashi M, Saitoh S. Lamivudine monotherapy for spontaneous severe acute exacerbation of chronic hepatitis B. J Gastroenterol Hepatol. 2005;20:426-432. [PubMed] |
| 5. | Laleman W, Wilmer A, Evenepoel P, Elst IV, Zeegers M, Zaman Z, Verslype C, Fevery J, Nevens F. Effect of the molecular adsorbent recirculating system and Prometheus devices on systemic haemodynamics and vasoactive agents in patients with acute-on-chronic alcoholic liver failure. Crit Care. 2006;10:R108. [PubMed] |
| 6. | Fitzpatrick E, Mitry RR, Dhawan A. Human hepatocyte transplantation: state of the art. J Intern Med. 2009;266:339-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Souza BS, Nogueira RC, de Oliveira SA, de Freitas LA, Lyra LG, Ribeiro dos Santos R, Lyra AC, Soares MB. Current status of stem cell therapy for liver diseases. Cell Transplant. 2009;18:1261-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901-904. [PubMed] |
| 9. | Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11-16. [PubMed] |
| 10. | Yannaki E, Anagnostopoulos A, Kapetanos D, Xagorari A, Iordanidis F, Batsis I, Kaloyannidis P, Athanasiou E, Dourvas G, Kitis G. Lasting amelioration in the clinical course of decompensated alcoholic cirrhosis with boost infusions of mobilized peripheral blood stem cells. Exp Hematol. 2006;34:1583-1587. [PubMed] |
| 11. | Theocharis SE, Papadimitriou LJ, Retsou ZP, Margeli AP, Ninos SS, Papadimitriou JD. Granulocyte-colony stimulating factor administration ameliorates liver regeneration in animal model of fulminant hepatic failure and encephalopathy. Dig Dis Sci. 2003;48:1797-1803. [PubMed] |
| 12. | Yannaki E, Athanasiou E, Xagorari A, Constantinou V, Batsis I, Kaloyannidis P, Proya E, Anagnostopoulos A, Fassas A. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol. 2005;33:108-119. [PubMed] |
| 13. | Salama H, Zekri AR, Zern M, Bahnassy A, Loutfy S, Shalaby S, Vigen C, Burke W, Mostafa M, Medhat E. Autologous hematopoietic stem cell transplantation in 48 patients with end-stage chronic liver diseases. Cell Transplant. 2010;19:1475-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Liu Y, Zhong Y, Zou Z, Xu Z, Li B, Ren X, Bai S, Wang L, Li X, Dai J. Features and clinical implications of hepatitis B virus genotypes and mutations in basal core promoter/precore region in 507 Chinese patients with acute and chronic hepatitis B. J Clin Virol. 2010;47:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Yang WB, Chen EQ, Bi HX, Bai L, Chen XB, Feng P, Tang H. Different models in predicting the short-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure. Ann Hepatol. 2012;11:311-319. [PubMed] |
| 16. | Huang K, Hu JH, Wang HF, He WP, Chen J, Duan XZ, Zhang AM, Liu XY. Survival and prognostic factors in hepatitis B virus-related acute-on-chronic liver failure. World J Gastroenterol. 2011;17:3448-3452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Di Campli C, Zocco MA, Saulnier N, Grieco A, Rapaccini G, Addolorato G, Rumi C, Santoliquido A, Leone G, Gasbarrini G. Safety and efficacy profile of G-CSF therapy in patients with acute on chronic liver failure. Dig Liver Dis. 2007;39:1071-1076. [PubMed] |
| 18. | Spahr L, Lambert JF, Rubbia-Brandt L, Chalandon Y, Frossard JL, Giostra E, Hadengue A. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology. 2008;48:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC, Sakhuja P, Sarin SK. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:505-512.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 291] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 20. | Khan AA, Parveen N, Mahaboob VS, Rajendraprasad A, Ravindraprakash HR, Venkateswarlu J, Rao P, Pande G, Narusu ML, Khaja MN. Management of hyperbilirubinemia in biliary atresia by hepatic progenitor cell transplantation through hepatic artery: a case report. Transplant Proc. 2008;40:1153-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Assmus B, Tonn T, Seeger FH, Yoon CH, Leistner D, Klotsche J, Schächinger V, Seifried E, Zeiher AM, Dimmeler S. Red blood cell contamination of the final cell product impairs the efficacy of autologous bone marrow mononuclear cell therapy. J Am Coll Cardiol. 2010;55:1385-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Garg H, Sarin SK, Kumar M, Garg V, Sharma BC, Kumar A. Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. Hepatology. 2011;53:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 23. | Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Diseases and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. [Diagnostic and treatment guidelines for liver failure]. Zhonghua Ganzangbing Zazhi. 2006;14:643-646. [PubMed] |
| 24. | Cui YL, Yan F, Wang YB, Song XQ, Liu L, Lei XZ, Zheng MH, Tang H, Feng P. Nucleoside analogue can improve the long-term prognosis of patients with hepatitis B virus infection-associated acute on chronic liver failure. Dig Dis Sci. 2010;55:2373-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Leber B, Spindelboeck W, Stadlbauer V. Infectious complications of acute and chronic liver disease. Semin Respir Crit Care Med. 2012;33:80-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |